|
Combined Educational & Scientific Session
ORGANIZERS: Jürgen K. Hennig, Ph.D., Roland Kreis, Ph.D. & Peter van Zijl, Ph.D.
Monday, 24 April 2017
| Room 314 |
16:15 - 18:15 |
Moderators: John Griffiths, Arend Heerschap |
Slack Channel: #s_mrs_molecular
Session Number: CE06
Overview
This session consists of two teaching lectures on the basics of metabolomics and metabolic fluxes measured using 13C MR spectroscopy, which provide the background for the scientific abstracts selected subsequently.
Target Audience
Scientists and clinicians interested in metabolism.
Educational Objectives
Upon completion of this course, participants should be able to:
-Describe the basics of metabolomics;
-Discuss applications of metabolomics of body fluids and tissues;
-Explain the basics of using 13C MR spectroscopy to measure metabolic fluxes; and
-Discuss applications of 13C tracers for the study of metabolism and their in vivo applications.
16:15
|
|
Metabolomic, tissue 
Peter Vermathen
Metabolomics denotes the comprehensive and simultaneous systematic profiling of metabolite levels through the study of biofluids and tissues. As such metabolomics is now considered an integral part of systems biology . Besides Mass Spectrometry, NMR Spectroscopy is the main analytical technique for simultaneous assessment of metabolites in biological fluids and tissues.
|
16:45
|
|
13C Metabolic Fluxes, not Hyperpolarized 
Douglas Rothman
An introduction to the use of 13C and 1H-13C MRS to measure metabolic fluxes in pre clinical models and clinical research studies will be presented. The presentation will have the following sections: 1. introduction to 13C MRS 2. use of 13C MRS to measure metabolite labeling 3. calculations of metabolic fluxes from metabolite labeling curves 4. applications to study metabolism in health and disease 5. application to study therapy. The main goals are to provide the audience with basic knowledge of how to perform and interpret 13C MRS measurements of metabolic fluxes and their potential for use in clinical research.
|
17:15
|
0327.
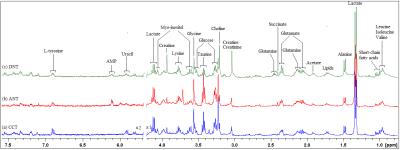 |
NMR-based metabolomics and metabolic pathway networks from patient-matched colorectal cancers, adjacent non-cancerous tissues and fecal extracts 
Yan Lin, Changchun Ma, Zhening Wang, Jiahao Liang, Yao Huang, Renhua Wu
This study aimed to profile paralleled metabolites of CRC tissues and adjacent non-cancerous tissues alongside pre- and post-operative stools from the same patients, to investigate how fecal metabolomic phenotypes correlate with the tumor tissue especially in a molecular pathology context. Our patient-matched cohort revealed a few overlapping discriminatory metabolites between the CRC and stool metabolomes, indicating the networks for metabolic pathway aberrations across both matrices. The altered metabolites potentially involved in the disruption of normal bacterial ecology, malabsorption of nutrients, increased glycolysis, TCA cycle and glutaminolysis, implying a Warburg effect for cell energy production required for rapid proliferation
|
17:27
 |
0328.
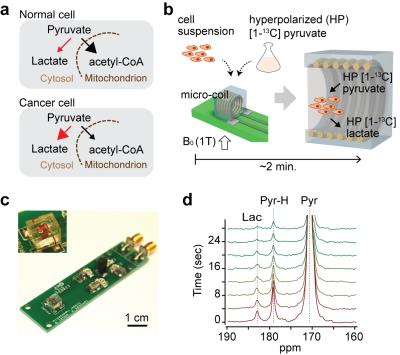 |
Hyperpolarized Micro-NMR for Metabolic Flux Analysis in Cancer Stem Cells and Rapid Assessment of Therapeutic Response 
Sangmoo Jeong, Roozbeh Eskandari, Sun Mi Park, Ralph Weissleder, Michael Kharas, Hakho Lee, Kayvan Keshari
Aberrant metabolic features of cancer cells are closely related to tumorigenesis and therapeutic response. Here, we report a sensitive magnetic resonance sensing platform, capable of analyzing metabolic fluxes in mass-limited samples. Termed hyperpolarized micromagnetic resonance spectrometer (HMRS), this platform achieved to characterize the metabolic flux in cancer stem cells in real-time and assess therapeutic responses much earlier than any changes in cell viability. This will become a versatile platform for rapid and sensitive exploration of metabolic dynamics in cancer.
|
17:39
|
0329.
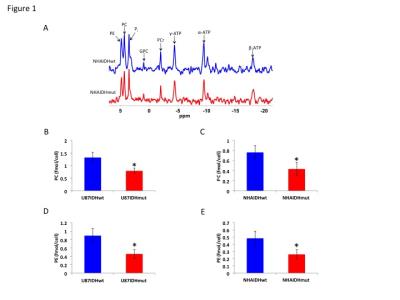 |
IDH1 mutation down-regulates choline and ethanolamine metabolism in gliomas - permission withheld
Pavithra Viswanath, Jose Izquierdo-Garcia, Joanna Phillips, Russell Pieper, Sabrina Ronen
Aberrant choline and ethanolamine metabolism with elevated phosphocholine (PC) and phosphoethanolamine (PE) levels has emerged as a hallmark of cancer. Interestingly, PC and PE levels are reduced in gliomas with the isocitrate dehydrogenase 1 (IDH1) mutation relative to wild-type tumors. Here, we investigated the mechanism behind the reduction in PC and PE levels in genetically-engineered cells and tumor xenografts. Our results indicate that mutant IDH1 gliomas down-regulate the activities of choline kinase and ethanolamine kinase, the enzymes involved in PC and PE synthesis. Reduced PC and PE levels constitute unique metabolic biomarkers and potential therapeutic opportunities in mutant IDH1 gliomas.
|
17:51
|
0330.
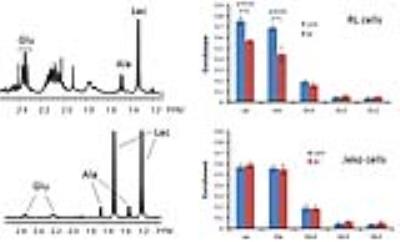 |
13C NMR metabolic flux analysis of mantle cell lymphoma cells to Bruton tyrosine kinase inhibitors - permission withheld
Seung-Cheol Lee, Alex Shestov, Stephen Pickup, Jeff Roman, Mariusz Wasik, Jerry Glickson
We analyzed various metabolic fluxes of mantle cell lymphoma cells upon Bruton tyrosine kinase signaling inhibitors using 13C NMR and a bonded cumomer modeling method and identified 1H NMR biomarkers translatable to clinic.
|
18:03
|
0331.
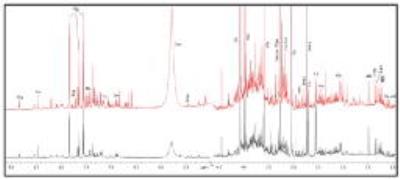 |
Identification of potential biomarkers for Parkinson’s disease by 1H NMR spectroscopy 
Senthil Kumaran, Sadhana Kumari, Vinay Goyal, SN Dwivedi, Achal Srivastava, Naranamangalam Jagannathan
We studied the metabolic profile of urine samples of patients with Parkinson’s disease (PD) and healthy controls (HC) using 700 MHz NMR spectrometer (Varian, M/s Agilent Technologies, USA). The data were processed using Vnmrj (version:2.3A) and binning data estimated using MestReNova software (version :10.0,Mestrelab Research, Spain). PLS-DA multivariate analysis was carried out using MetaboAnalyst (ver.3.0), a web-based metabolomics data processing tool to evaluate significance of metabolites in PD with respect to HC. We observed elevated levels of lactate, tryptophan, glycine and reduced levels of citrate, leucine, isoleucine (t-test, p<0.05), suggestive of several metabolic abnormalities, as mitochondrial dysfunction and reduced bioenergetics efficiency in PD patients.
|
|






