Monday, 24 April 2017
| Room 314 |
08:15 - 10:15 |
Moderators: Pinar Özbay, Wei Huang |
Slack Channel: #s_contrastmechanisms
Session Number: O50
08:15
|
0067.
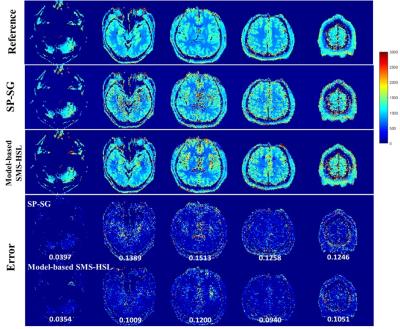 |
Accelerated MR Parameter Mapping Exploiting Model-Based Simultaneous Multi-Slice Reconstruction with Hankel Subspace Learning: Application to T1 Quantification 
Sugil Kim, Suhyung Park, Jaeseok Park
MR parameter mapping has been potentially of great value in diagnosing pathological diseases, but is difficult to be translated to clinical applications due to prohibitively long imaging time. It was recently shown in [1-4] that simultaneous multi-slice (SMS) imaging is highly efficient in reducing imaging time while well maintaining SNR. In this work, we propose a novel, model-based SMS reconstruction approach with Hankel subspace learning (Model-based SMS-HSL) for highly accelerated MR parameter mapping under the hypothesis that the null space in the spatial dimension, which filters out slices of no interest, is time-invariant in the parameter dimension while the dimension of temporal basis, which is found from signal evolution models, is limited.
|
08:27
|
0068.
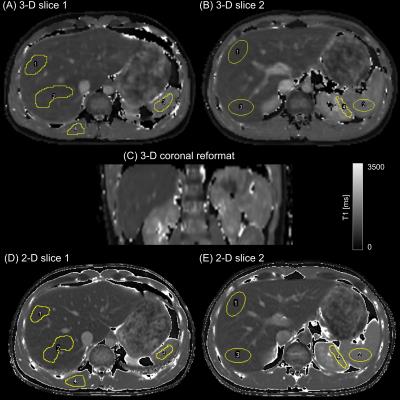 |
Single Breath-hold Abdominal T1 Mapping using 3-D Cartesian Sampling and Spatiotemporally Constrained Reconstruction 
Felix Lugauer, Jens Wetzl, Christoph Forman, Manuel Schneider, Berthold Kiefer, Dominik Nickel, Andreas Maier
Volumetric T1 mapping in the abdomen is desirable for whole liver assessment of hepatic diseases. In case of breath-hold imaging, accurate but time-consuming methods that sample the relaxation curve (IR or Look-Locker) are restricted to few slices only. To address these limitations, sparse Cartesian sampling with spatiotemporal incoherence is utilized to render 3-D Look-Locker within a single breath-hold possible. We demonstrate feasibility in both phantom and in-vivo measurements. The proposed method shows high agreement with a 2-D reference acquisition and enables an accurate mapping for a wide T1 range, including very low values due to its high temporal resolution.
|
08:39
 |
0069.
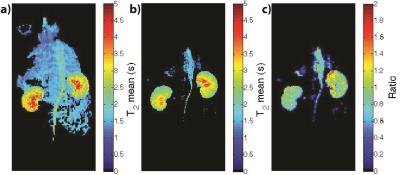 |
Accelerating High Resolution Hyperpolarized 13C T2 Mapping Using a Local Low Rank plus Sparse Reconstruction 
Eugene Milshteyn, Galen Reed, Cornelius von Morze, Zihan Zhu, Jeremy Gordon, Daniel Vigneron
Hyperpolarized 13C probe development has allowed in vivo monitoring of different physiological processes relating to various diseases, including cancer and diabetes. Each new probe is typically characterized with polarization and T1measurements, but T2 is also an important parameter for optimal sequence design, including progressive flip angle schemes. To improve the spatiotemporal resolution of T2 mapping sequences and subsequent multi-exponential analysis, this project investigated using a local low rank plus sparse reconstruction for 2-fold acceleration of in vivo T2 mapping with the bSSFP sequence.
|
08:51
 |
0070.
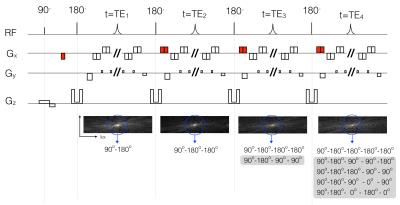 |
Ultrafast T2 mapping using echo-split GRASE acquisition and parametric POCSMUSE reconstruction 
Mei-Lan Chu, Hing-Chiu Chang, Koichi Oshio, Nan-kuei Chen
Our novel ultrafast T2 mapping framework, which uniquely integrates echo-split GRASE acquisition and parametric POCSMUSE reconstruction, has the following major advantages. First, parametric T2 map and high-quality multi-contrast images can be derived from a single set of single-shot GRASE data, with inherently low susceptibility to motion artifacts. Second, contamination of stimulated and other high order echoes is minimized in the echo-split GRASE scans. Third, T2 relaxation times can be accurately measured by the parametric POCSMUSE algorithm, which incorporates multiplexed parallel MR reconstruction and multi-echo-pathway signal modeling into a unified procedure.
|
09:03
|
0071.
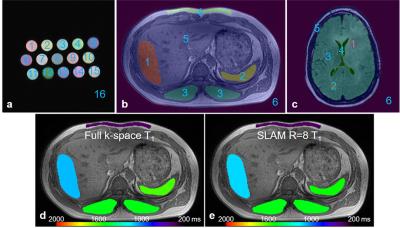 |
Ultrafast compartmental relaxation time mapping with linear algebraic modeling 
Yi Zhang, Xiaoyang Liu, Jinyuan Zhou, Paul Bottomley
Image contrast afforded by tissue longitudinal (T1) and transverse (T2) relaxation times is central to the success of modern MRI. Here, a recently-proposed ‘spectroscopy with linear algebraic modeling’ (SLAM) method is adapted to dramatically accelerate relaxation time imaging at 3 Tesla in phantoms, the abdomens of six volunteers and in six brain tumor patients. SLAM is validated by omitting up to 15/16ths (94%) of the data acquired retroactively from inversion recovery and multi-echo spin-echo sequences, and proactively applied to accelerate abdominal and brain tumor T1 and T2 measurements by up to 16-fold in humans.
|
09:15
|
0072.
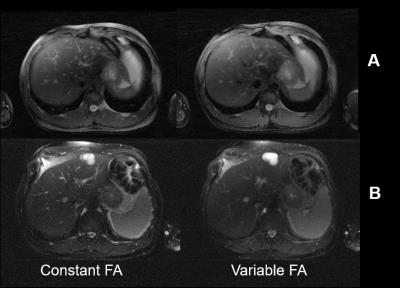 |
Variable Flip Angle Radial Turbo Spin Echo Technique for Abdominal T2 Mapping 
Mahesh Bharath Keerthivasan, Manojkumar Saranathan, Jean-Philippe Galons, Diego Martin, Ali Bilgin, Maria Altbach
The estimation of T2 relaxation times within lesions can provide a quantitative method of classifying abdominal neoplasms. Accelerated T2 mapping approaches have been proposed using the Radial TSE (RADTSE) sequence, where high resolution images at multiple TEs are reconstructed from data acquired in a single breath hold. However, the slice coverage for TSE based breath-held imaging is SAR restricted, motivating the need to reduce the refocusing flip angle. We present a variable refocusing flip angle RADTSE sequence designed to optimize the signal evolution for T2 mapping in the abdomen with reduced SAR, thereby increasing the slice coverage.
|
09:27
|
0073.
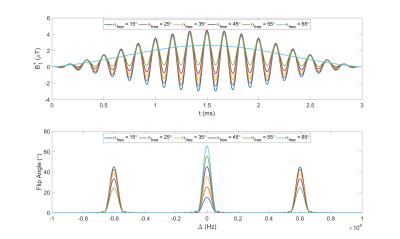 |
Robust VFA relaxometry by Continuous Saturation of Magnetization Transfer (CSMT) effects with Non-selective Multi-Band pulses. 
Rui Pedro A. G. Teixeira, Anthony N. Price, Ana A. Baburamani, Shaihan J. Malik, Joseph V. Hajnal
Variable Flip Angle (VFA) relaxometry methods have recently been shown to be sensitive to magnetization transfer (MT) induced bias. Common description of this effect relies on a two-pool model (restricted macromolecular pool & visible free water pool). Current practice to restrict influence of MT consists in stretching of RF pulse durations in order to minimize/counter-balance the effect of macromolecular exchange for different flip angle measurements. This work proposes to minimize the estimation bias by using constant saturation MT pulses that simultaneously excite the free-water pool and saturate the restricted-pool creating constant RF-saturation conditions independently of the flip angle (FA) applied.
|
09:39
|
0074.
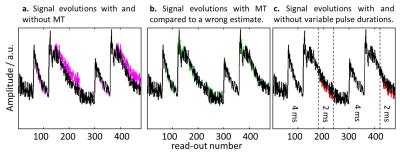 |
Mitigating the Effect of Magnetization Transfer in Magnetic Resonance Fingerprinting 
Tom Hilbert, Tobias Kober, Tiejun Zhao, Tobias Block, Zidan Yu, Jean-Philippe Thiran, Gunnar Krueger, Daniel Sodickson, Martijn Cloos
Magnetic Resonance Fingerprinting (MRF) is a powerful technique for the quantification of relaxation parameters, and ideally provides correct estimates independent from the sequence used. In this work, we show that the quantification can be influenced by the pulse duration or, equivalently, the pulse bandwidth. This behavior, which we hypothesize to be related to magnetization transfer (MT) effects, is shown for the PnP-MRF sequence. We propose a first approach to encode MT effects in the MRF sequence and to model MT effects in the reconstruction, showing that this mitigates the bias in the resulting relaxation estimates.
|
09:51
|
0075.
 |
Clinically viable FAST-T2 based whole brain myelin water content mapping: T1 validation and initial MS lesion study 
Thanh Nguyen, Yihao Yao, Pascal Spincemaille, Eric Morris, Susan Gauthier, Yi Wang
The objectives of this study were to validate the accuracy of FAST-T1 mapping required for myelin water content (MWC) mapping, and to demonstrate the feasibility of fast MWC mapping in MS patients. FAST-T1 provides whole brain T1 map in 3 min, which was in excellent agreement with that obtained with the reference IR-FSE method. MWC mapping in 20 MS patients showed a consistent increase in water content in MS lesions (10.7% on average), accompanied by 62.8% increase in T1 and 44.6% decrease in MWC when compared to the contralateral NAWM.
|
10:03
|
0076.
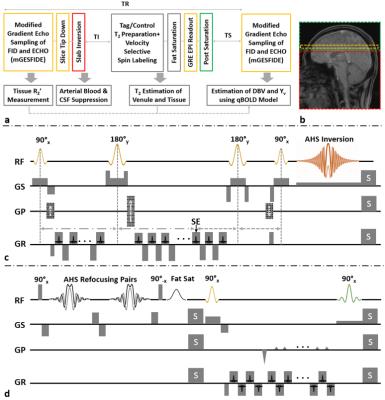 |
Quantitative BOLD With Interleaved Acquisitions for Estimation of Extravascular $$$R_2’$$$ and Intravascular $$$R_2$$$ With Phase-Sensitive CSF Suppression 
Hyunyeol Lee, Cheng Li, Erin Englund, Felix Wehrli
In the qBOLD technique, the accuracy of local deoxygenated blood volume and hemoglobin oxygen saturation (Yv) maps is potentially degraded due to high coupling of the two parameters in the model. As an alternative, the QUIXOTIC method measures local Yv by selectively capturing venular spins via T2-prepared velocity-selective-spin-labeling. However, CSF signals, if not suppressed, may impair accuracy of venular blood T2 estimation. In this work, extravascular R2’ and intravascular R2 mapping methods are interleaved to reduce estimation uncertainty in the qBOLD model while the accuracy of preliminary venular T2 estimates from the latter is further enhanced via phase-sensitive CSF suppression.
|
|












