Monday, 24 April 2017
| Room 311 |
16:15 - 18:15 |
Moderators: Susie Huang, Itamar Ronen |
Slack Channel: #s_diffusion
Session Number: O70
16:15
 |
0277.
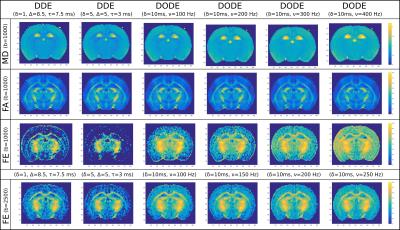 |
Time dependence of microscopic anisotropy in the mouse brain measured with double oscillating diffusion encoding (DODE) MRI 
Andrada Ianus, Sune Jespersen, Daniel Alexander, Ivana Drobnjak, Noam Shemesh
Time dependence of microscopic anisotropy measured with diffusion MRI can reveal the cellular eccentricity at different lengths scales, which is an important step towards the goal of non-invasive characterization of tissue microstructure. Diffusion sequences which vary the gradient orientation within one measurement can probe microscopic anisotropy, regardless of the macroscopic tissue configuration. Here we employ the newly proposed Double Oscillating Diffusion Encoding (DODE) sequences, consisting of two independent trains of oscillating gradients which can have different orientations, in order to measure the time dependence of microscopic anisotropy in the mouse brain.
|
16:27
|
0278.
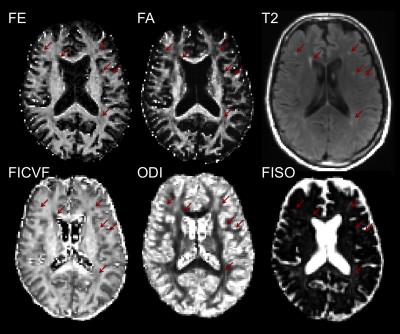 |
Comparison of Double Diffusion Encoding and NODDI 
Grant Yang, Qiyuan Tian, Christoph Leuze, Jennifer McNab
In this study, we compare fractional eccentricity (FE) measured by double diffusion encoding (DDE) to NODDI estimates of neurite density and orientation distribution in six normal subjects and one subject with benign T2 hyperintensities. The results of the comparison support the hypothesis that FE is independent of fiber orientation and correlates strongly with intracellular volume fraction.
|
16:39
|
0279.
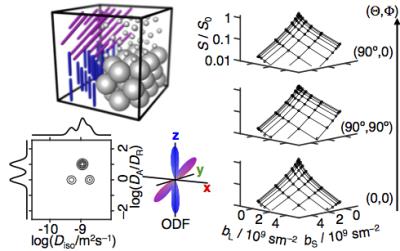 |
Diffusion tensor distribution imaging 
Daniel Topgaard
Diffusion MRI is an excellent method for detecting subtle changes of brain microstructure, but rarely gives unambiguous information about whether the observations originate from variations in cell density, size, shape, orientation, or any combination thereof. Capitalizing on our recent pulse sequences with data acquisition as a function of both the spherical and the conventional linear components of the diffusion encoding tensor b, we here introduce and demonstrate a method to quantify the composition of a heterogeneous voxel as a multidimensional distribution of diffusion tensors where the information about size, shape, and orientation is cleanly separated in the respective dimensions of the distribution. When transferred to a neuroimaging context, our method will allow for unconstrained estimation of fiber bundle orientation distributions and radial and axial diffusivities, as well as fractions of extracellular water and cerebrospinal fluid.
|
16:51
 |
0280.
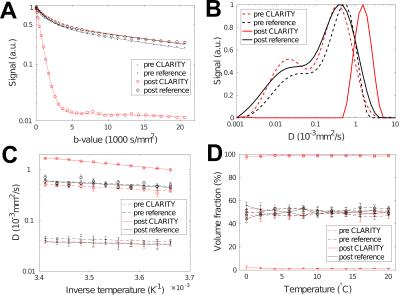 |
Validation of the two-pool diffusion model in post-mortem white matter using the CLARITY method 
Jakob Georgi, Markus Morawski, Carsten Jäger, Harald Möller
Water diffusion in tissues is known to be non-Gaussian. Moreover, two different water regimes have been found in brain tissue and assigned to a bulk-like compartment and water in contact with macromolecules. Here, we investigate the influence of membranes in post-mortem white matter, which are assumed to be responsible for the second pool observed in MR-diffusion measurements. Using a newly developed CLARITY method, which removes lipids from brain tissue while keeping the brain structure intact, we found that the slow compartment vanished while the mobility of the fast pool increased, which directly demonstrates the influence of membranes on water dynamics.
|
17:03
|
0281.
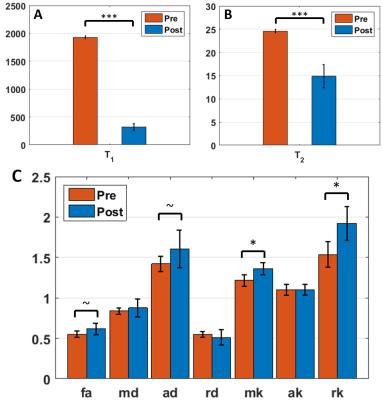 |
Intra- and extra-axonal axial diffusivities in the white matter: which one is faster? 
Ileana Jelescu, Nicolas Kunz, Analina Da Silva, Rolf Gruetter
The typical two-compartment model of diffusion in the white matter is associated with two plausible solutions, the choice between which relies on whether intra-axonal or extra-axonal axial diffusivity is faster. Here we use an intracerebroventricular perfusion of gadolinium in the rat brain to suppress the extra-cellular signal. Diffusion measurements before and after perfusion show a mild increase in axial diffusivity post-perfusion, which suggests intra-axonal diffusivity is higher than extra-axonal axial diffusivity. This can help solve the current indetermination in parameter estimation and allow diffusion models to regain their claimed specificity.
|
17:15
|
0282.
 |
Universal power-law scaling of water diffusion in human brain defines what we see with diffusion MRI 
Jelle Veraart, Els Fieremans, Dmitry Novikov
Here we identify a universal power-law scaling behavior of the diffusion MRI signal on a clinical scanner. This specific functional form provides a defining signature of water confined within narrow sticks establishing that exchange between intra- and extra-axonal water is not relevant, and the fraction of fully restricted water is negligible in the clinically accessible regime. The observed scaling for the first time in vivo validates the key ingredient specific to the microstructural models of MRI signal from neuronal tissue and enables the in vivo quantification of intra-axonal properties.
|
17:27
|
0283.
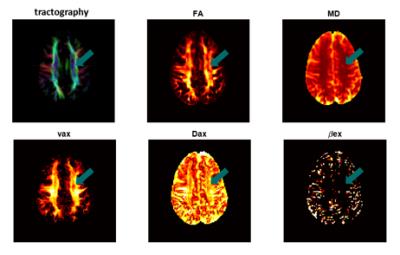 |
Multi-compartment microscopic diffusion imaging with oscillating gradients: simulation validation and application in multiple sclerosis patients - permission withheld
Hua Li, Enrico Kaden, Daniel Alexander, John Gore, Bagnato Francesca, Junzhong Xu
Microscopic diffusion imaging using spherical mean technique (SMT) and oscillating gradient spin echo (OGSE) was applied in multiple sclerosis patients, along with computer simulation validation. The results suggested that there are significant decreases of axon volume fraction in multiple sclerosis patients compared with contralateral normal tissue.
|
17:39
|
0284.
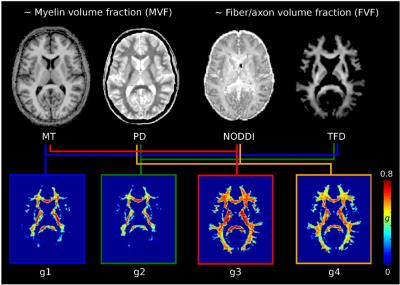 |
Comparing in vivo MR g-ratio mapping methods: accuracy and precision at the group level 
Isabel Ellerbrock, Siawoosh Mohammadi
The g-ratio, the ratio between the inner and outer diameter of a myelinated axon, is of great neuroscientific interest because it is a relative measure of axonal myelination and functionally linked to conduction velocity. In vivo g-ratio mapping has been recently suggested using a flexible biophysical model that relates the microscopic g-ratio, only accessible by histology, to MRI biomarkers for the myelin and fiber compartment. This study investigates the question which MRI biomarker is optimal for MR g-ratio mapping concerning precision (determined by scan-rescan reproducibility) and accuracy (assessed by comparability to previous in vivo and the ex vivo results).
|
17:51
|
0285.
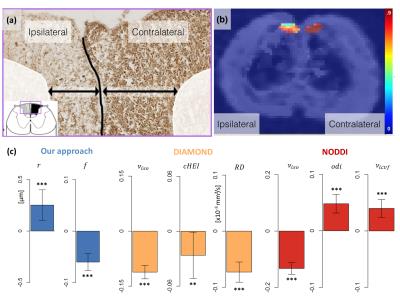 |
Microstructure imaging from a dictionary of Monte Carlo signals: assessment on a rat model of Wallerian degeneration 
Gaëtan Rensonnet, Benoît Scherrer, Simon Warfield, Benoît Macq, Maxime Taquet
We estimate microstructural features of the nervous tissues from diffusion-weighted MRI by using sparse optimization techniques on a dictionary of pre-computed Monte Carlo signals, which more faithfully describe the complex diffusion process in the extra-axonal space of the white matter. The method is validated on synthetic data including single and crossing fibers and on an in vivo rat spinal cord model of Wallerian degeneration. We obtain in vivo microstructural estimates that can be directly related to histological evidence whereas the traditional closed-form formula models DIAMOND and NODDI yield results that are more challenging to interpret physically.
|
18:03
|
0286.
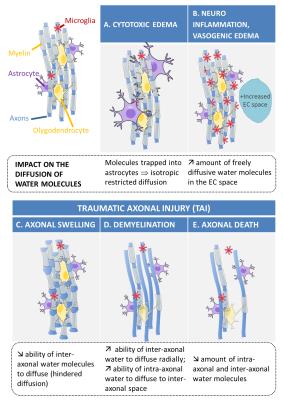 |
Diffusion compartment imaging reveals microstructural injuries in a mouse model of mild traumatic brain injury 
Benoit Scherrer, Jianhua Qiu, Jumana Hashim, Onur Afacan, Yaotang Wu, Michael Marcotrigiano, Simon Warfield, Rebekah Mannix
Although about 30% of patients with mild traumatic brain injury (mTBI) suffer prolonged symptoms after injury1, conventional anatomic magnetic resonance imaging (MRI) has not proven useful in diagnosing or predicting outcomes after mTBI. In this work we evaluated a novel technique, diffusion compartment imaging (DCI), with a mouse model of mTBI that enables study of mTBI under controlled conditions. We compared DCI and diffusion tensor imaging (DTI) changes to histopathological observations in two injury conditions (with and without persistent functional deficits). Our results suggest that, unlike DTI, DCI detects specific evidence of traumatic axonal injury. Moreover, DCI detects changes only in mice with persistent functional deficits.
|
|











