Myocardial Tissue Characterization
Myocardial Tissue Characterization
Oral
Oral
Cardiovascular
Tuesday, 14 May 2019
| Room 513D-F | 08:15 - 10:15 | Moderators: Sonia Nielles-Vallespin, Jiaxin Shao |
| 08:15 |
0400. 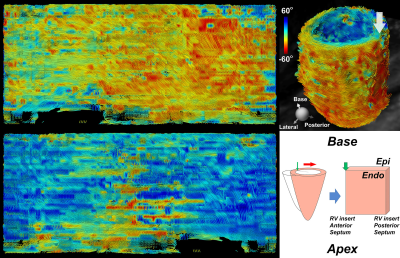 |
Free Breathing Isotropic Cardiac Diffusion Tensor MRI of the Left Ventricle Using M2-gSlider: Unfolding the Fiber Architecture of the Human Heart
Christopher Nguyen, Timothy Reese, Congyu Liao, William Kostis, Marcel Jackowski, Kawin Setsompop, Choukri Mekkaoui
Free breathing isotropic cardiac diffusion tensor MRI (DT-MRI) of the entire left ventricle was achieved by combining two recently-developed technologies: second moment (M2) motion compensated spin echo encoding and generalized slice dithered enhanced resolution (gSlider). M2-gSlider can address cardiac motion-induced signal loss under free breathing and can achieve isotropic spatial resolution of 2.5 mm. With spatial resolution three times that of conventional cardiac DT-MRI, the precision of tractography-based indices can be improved. Furthermore, isotropic acquisition eases the interpretation of myocardial fiber architecture including in an “unfolded” representation, depicting both circumferential and longitudinal microstructure in a planar format.
|
08:27 |
0401. 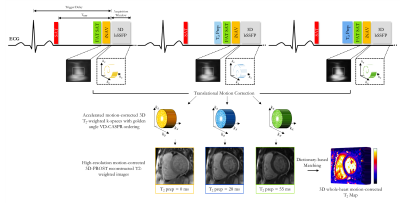 |
Fast and Accurate Free-Breathing Whole-Heart 3D T2 Mapping
Aurelien Bustin, Giorgia Milotta, Radhouene Neji, René Botnar, Claudia Prieto
T2 mapping is a promising quantitative imaging technique for the detection of myocardial edema. Conventionally, T2 mapping is performed using T2-prepared single-shot 2D acquisitions, acquiring multiple slices in several breath-holds. While showing high accuracy and reproducibility, breath-holding limits achievable spatial resolution and heart coverage and can be challenging in very sick patients. Here we propose a free-breathing whole-heart 3D T2 mapping technique with high isotropic spatial resolution in a clinically feasible scan time. This is achieved by combining an accelerated T2-prepared acquisition with patch-based reconstruction and dictionary-based signal matching. Feasibility of the proposed method was investigated in a standardized T1/T2 phantom and healthy subjects.
|
| 08:39 |
0402. 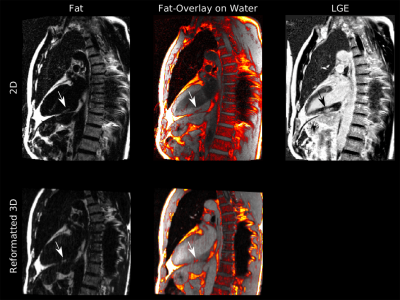 |
Motion-corrected 3D high-resolution fat-water imaging of the heart
Christoph Kolbitsch, Johannes Mayer, Alberto Cipriani, Edyta Blaszczyk, Jeanette Schulz-Menger, Tobias Schaeffter
Fat infiltration in the myocardium is of great clinical interest as potential predictor of poor prognosis in patients with either ischemic or non-ischemic cardiac diseases. Commonly, 2D chemical shift encoded MR methods are used to obtain fat-water separated images. Nevertheless, fat infiltrations are often small and hence could be missed due to poor slice resolution. Here we propose a free-breathing 3D whole-heart fat-water imaging approach with an isotropic resolution of 1.5mm3, which uses respiratory motion correction and retrospective cardiac gating. The approach was evaluated in five patients demonstrating accurate visualisation of the heart and small fat infiltrations in the myocardium.
|
| 08:51 |
0403. 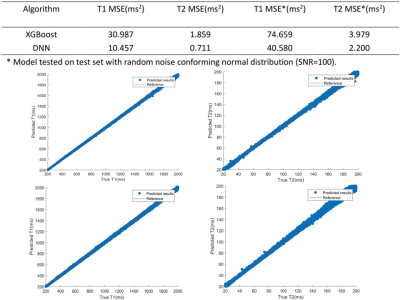 |
Fast T1, T2 evaluation with machine learning for quantitative cardiac MRI
Xianghao Zhan, Jiaxin Shao, Peng Hu
Quantitative T1/T2 mapping provides important cardiovascular prognostic value. Conventional dictionary-matching based methods are time consuming for cardiac T1/T2 mapping as the dictionary need to be generated on-line. In this work, we propose to use machine learning algorithms for faster T1/T2 prediction. Bloch equation simulation was used to generate training data. The XGBoost and DNN models were evaluated and compared based on simulation, phantom and in vivo studies. Results demonstrated that using the machine learning approach can generate cardiac T1 and T2 maps much faster while generating similar T1 and T2 values compared to the conventional dictionary-matching approach.
|
| 09:03 |
0404. 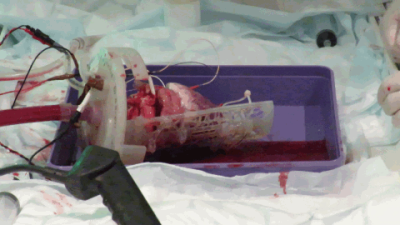 |
Diffusion tensor cardiovascular magnetic resonance in a Langendorff perfused beating porcine heart
Andrew Scott, Timothy Jackson, Zohya Khalique, Margarita Gorodezky, Ben Pardoe, Domenico Bruno, Rasheda Chowdhury, Ferreira Pedro, Sonia Nielles-Vallespin, Lale Begum, Malte Roehl, Padmini Sarathchandra, Jan Rose, Denis Doorly, Dudley Pennell, Raimondo Ascione, Ranil de Silva, David Firmin
We have developed an MRI compatible Langendorff perfused beating isolated porcine heart model. Hearts are harvested and transported to a remote imaging centre, avoiding the need for onsite pre-clinical facilities. The model allows comparison of beating and arrested data in equivalent cardiac states. We performed cine imaging, parametric mapping and diffusion tensor cardiovascular magnetic resonance in the beating and arrested hearts. After MRI, co-localised 3D histology is performed.
|
| 09:15 |
0405.  |
Combined Cardiac CINE and T1, T2, and M0 Mapping with MR Fingerprinting
Jesse Hamilton, Mark Griswold, Nicole Seiberlich
A method is introduced for combined cardiac CINE and T1, T2, and M0 quantification using MR Fingerprinting throughout the cardiac cycle. Data are acquired continuously using an MRF acquisition and binned by cardiac phase using an external ECG. Undersampled MRF images from all TRs are non-rigidly registered to a consistent cardiac phase before pattern matching in order to generate CINE T1, T2, and M0 maps. CINE-MRF has a breathhold duration (10s) and temporal resolution (25 cardiac phases) similar to conventional CINE scans, while providing both relaxation time measurements and functional information.
|
09:27 |
0406. 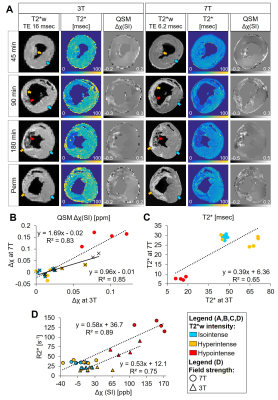 |
Magnetic susceptibility and T2* of myocardial reperfusion injury at 3 and 7 T
Brianna Moon, Srikant Kamesh Iyer, PhD, Nicholas Josselyn, Akito Imai, MD, Keitaro Okamoto, MD, Yoshiaki Saito, MD, James Pilla, PhD, Joseph Gorman III, MD, Robert Gorman, MD, Giovanni Ferrari, PhD, Yuchi Han, MD, Harold Litt, MD-PhD, Walter Witschey, PhD
Intramyocardial hemorrhage is a frequent complication of acute myocardial infarction (MI) after reperfusion therapy. This study investigated the relationship between T2* and susceptibility and how they are affected by magnetic field strength. We compare cardiac quantitative susceptibility mapping (QSM) and T2* at 3 and 7 T and demonstrate improved detection of hemorrhage and infarct regions at 7 T in a large animal model correlating with infarct pathophysiology.
|
| 09:39 |
0407. 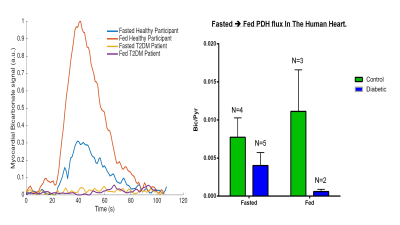 |
Hyperpolarised 13C MR Spectroscopy Demonstrates Impaired PDH flux In the Diabetic Human Heart, Correlating with Impaired Energetics, Relaxation and Increased Myocardial Lipid Content: A Multi–Nuclear Spectroscopy Study.
Andrew Apps, Justin Lau, Jack Miller, Mark Peterzan, Andrew Lewis, Michael Dodd, Angus Lau, Ferenc Mozes, Oliver Rider, Stefan Neubauer, Damian Tyler
Hyperpolarised 13C MRS offers unparalleled opportunities for studying in-vivo, real time metabolism. In our current study we demonstrate how the technique easily demonstrates early pathological changes in PDH flux in the diabetic heart, and is complementary to other spectroscopic and imaging techniques, in defining the metabolic and structural changes characterising the disease.
|
09:51 |
0408. 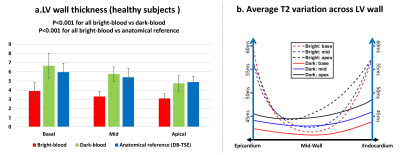 |
Cardiac dark-blood T2 mapping increases effective myocardial thickness by 35-75% for T2 evaluation in healthy subjects and patients
Chenxi Hu, Steffen Huber, Vinh Nguyen, Lauren Baldassarre, Hamid Mojibian, Dana Peters
Cardiac dark-blood T2 mapping is an emerging technique to measure myocardial T2 with simultaneous suppression of blood pool and pericardial fat. In this work, we compared dark-blood T2 mapping with conventional bright-blood T2 mapping in terms of accuracy, precision, and effective myocardial thickness, in 8 healthy subjects and 7 patients. We found similar accuracy and precision between the two T2 mapping methods, but a largely improved effective myocardial thickness with dark-blood T2 mapping, due to reduced partial-voluming. The increased thickness may improve the accuracy of T2 in thin-walled structures for clinical evaluation.
|
10:03 |
0409. 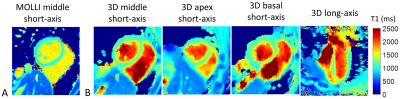 |
Free-running 3D Whole Heart Myocardial T1 Mapping with High Isotropic Spatial Resolution
Haikun Qi, Olivier Jaubert, Aurelien Bustin, Gastao Cruz, Huijun Chen, René Botnar, Claudia Prieto
Myocardial T1 mapping provides quantitative tissue characterization for the assessment of various cardiomyopathies. However, currently available myocardial T1 mapping techniques still have several limitations such as insufficient coverage, low spatial resolution, and the need of acquiring the data under multiple breath-holds. To overcome these problems, here we propose a free-running (free-breathing, no ECG gating) 3D whole heart T1 mapping technique with high isotropic spatial resolution. This approach allows for myocardial T1 mapping at arbitrary cardiac phases, enabling high-resolution dynamic T1 maps. The feasibility of the proposed sequence was validated against conventional methods in phantom and five healthy subjects.
|
 Back to Program-at-a-Glance |
Back to Program-at-a-Glance |  Back to Top
Back to Top