Scientific Session
Multiple Sclerosis & Myelin
| Monday Parallel 2 Live Q&A | Monday, 10 August 2020, 13:45 - 14:30 UTC | Moderators: Michael Barnett & Susie Huang |
0037.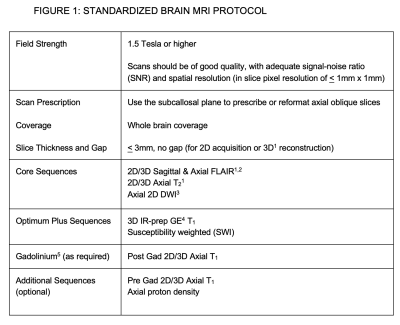 |
Developing a Universally Useful, Useable and Used Standardized MRI Protocol for Patients with Multiple Sclerosis
David K.B. Li1, Frederik Barkhof2, Scott Newsome3, June Halper4, Lori Saslow4, Brenda Banwell5, Laura Barlow6, Kathleen Costello7, Peter Damiri8, Marilyn Maes9, Sarah Morrow10, Jiwon Oh11, Friedemann Paul12, Patrick
Quarterman13, Daniel Reich14, Jason Shewchuk15, Russell Shinohara16, Wim Van Hecke17, Kim van de Ven18, Amy Verrinder9, Mitchell Wallin19, Jerry Wolinsky20, and Anthony Traboulsee21
1Radiology, University of British Columbia, Vancouver, BC, Canada, 2VU University Medical Center, Amsterdam, Netherlands, 3Johns Hopkins University, Baltimore, MD, United States, 4Consortium of MS Centers, Hackensack, NJ, United States, 5Children's Hospital of Philadelphia, Philadelphia, PA, United States, 6UBC MRI Research Center, University of British Columbia, Vancouver, BC, Canada, 7National MS Society, New York, NY, United States, 8Multiple Sclerosis Association of America, Cherry Hill, NJ, United States, 9Cortechs Labs, San Diego, CA, United States, 10London MS Clinic, Western University, London, ON, Canada, 11University of Toronto, Toronto, ON, Canada, 12NeuroCure Clinical Research Center, Charité Universitätsmedizin, Berlin, Germany, 13General Electric Healthcare, Milwaukee, WI, United States, 14Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, MD, United States, 15University of British Columbia, Vancouver, BC, Canada, 16University of Pennsylvania, Philadelphia, PA, United States, 17icometrix, Leuven, Belgium, 18BIU MR, Philips Healthcare, Eindhoven, Netherlands, 19VA MS Medical Center of Excellence-East, Washington, DC, United States, 20McGovern Medical School, University of Texas Health Science Center, Houston, TX, United States, 21Neurology, University of British Columbia, Vancouver, BC, Canada
Standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis (MS) have been available for over a decade. These guidelines are useful and useable, but not yet widely used. An expert panel with representatives from CMSC, NAIMS, NMSS, MSAA and MRI vendors updated the MRI protocol with the vision of creating international guidelines to be universally adopted as the standard of care for use of MRI in MS. Novel methods of disseminating information to payers, patient groups, MRI Centers and MRI vendors so that the MRI protocol will be used were discussed and will be explored.
|
|
0038.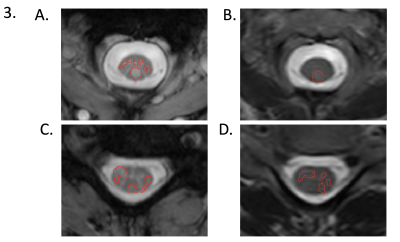 |
Comparison of mFFE & Axial T2-Weighted Fast-Spin-Echo Sequences for Lesion Detection in Low-Disability Multiple Sclerosis Patients
Mereze Visagie1, Atlee Witt1, Sanjana Satish1, Shekinah Malone2, Anna Combes1, Kristin P O'Grady1,3, Dylan Lawless1,4, Francesca Bagnato5, Colin McKnight3, and Seth A Smith1,3
1Vanderbilt University Institute of Imaging Science, Nashville, TN, United States, 2Meharry Medical College, Nashville, TN, United States, 3Radiology & Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 4Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 5Clinical Neurology, Vanderbilt University Medical Center, Nashville, TN, United States
In multiple sclerosis (MS), detection of lesions in the spinal cord with MRI is important for diagnosis and monitoring of disease progression. Despite improved clinical MRI sequences, motion and pulsation artifact remain a challenge for small lesion identification. We sought to compare sensitivity for lesion detection between multi-echo gradient echo (mFFE) and T2-weighted fast-spin-echo (T2-FSE) sequences at 3T in 16 relapsing-remitting MS patients with low disability. By comparing lesion fraction and average lesion burden, we demonstrated that mFFE has greater sensitivity for spinal cord lesions than T2-FSE.
|
|
0039.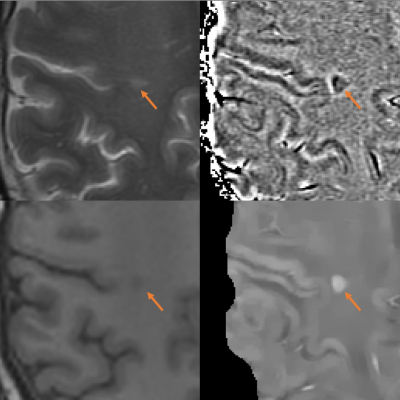 |
A comparison of phase image and quantitative susceptibility mapping in identifying inflammation in chronic multiple sclerosis lesions
Xianfu Luo1,2, Ulrike W. Kaunzner3, Thanh D. Nguyen1, Yeona Kang4, Elizabeth Sweeney5, Weiyuan Huang1, Yi Wang1, and Susan Gauthier3
1Department of Radiology, Weill Medical College of Cornell University, New York, NY, United States, 2Department of Radiology, Northern Jiangsu People's Hospital, Yangzhou, China, 3Department of Neurology, Weill Medical College of Cornell University, New York, NY, United States, 4Department of Radiology/Nuclear Medicine, Weill Medical College of Cornell University, New York, NY, United States, 5Department of Healthcare Policy and Research, Weill Medical College of Cornell University, New York, NY, United States
Both MR phase imaging and quantitative susceptibility mapping (QSM) are used to assess the presence of chronic active multiple sclerosis lesions. It is important to evaluate which measure can detect ongoing inflammation in chronic active lesions most accurately. This study combined PK11195-PET with QSM versus phase imaging, and demonstrated that QSM can detected higher uptake of PK11195, as compared to phase imaging.
|
|
1254.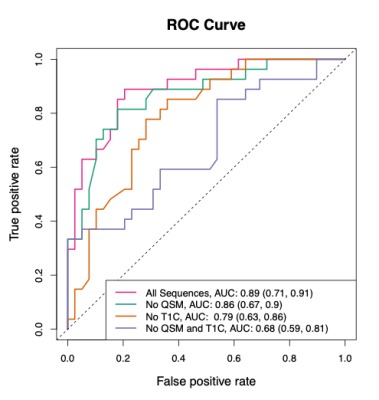 |
Estimation of Multiple Sclerosis Lesion Age without Gadolinium using Quantitative Susceptibility Maps
Elizabeth Margaret Sweeney1, Thanh Nygen1, Amy Kuceyeski1, Sarah Ryan 2, Shun Zhang1, Yi Wang1, and Susan Gauthier1
1Weill Cornell, New York, NY, United States, 2University of Colorado Denver, Denver, CO, United States
We propose a method to estimate multiple sclerosis (MS) lesion age (less than or greater than a year old) using non-gadolinium magnetic resonance imaging. The method utilizes the less invasive Quantitative Susceptibility Map. Radiomic features are calculated over a lesion and a random forest classification model is used. In a validation set, the model has an AUC of 0.79 (95% CI: [0.63, 0.86]) and an accuracy of 0.73 (95% CI: [0.60, 0.80]). This method can be used to aid in the diagnosis of MS, as part of the diagnostic criteria is to show lesion dissemination in time.
|
|
 |
0040.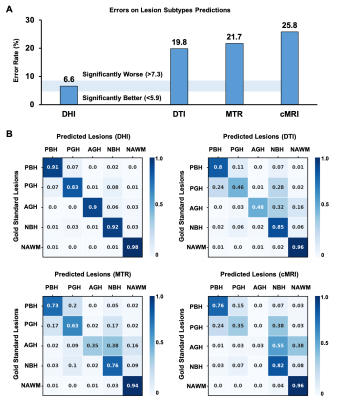 |
Diffusion Histology Imaging Classifies Lesions in Multiple Sclerosis
Zezhong Ye1, Ajit George1, Anthony T. Wu2, Xuan Niu1, Joshua Lin3, Gautam Adusumilli4, Robert T. Naismith4, Anne H. Cross4, Peng Sun1, and Sheng-Kwei Song1
1Radiology, Washington University School of Medicine, Saint Louis, MO, United States, 2Biomedical Engineering, Washington University, Saint Louis, MO, United States, 3Keck School of Medicine, The University of Southern California, Los Angeles, CA, United States, 4Neurology, Washington University School of Medicine, Saint Louis, MO, United States
MS lesions have heterogeneous pathology, including inflammation, demyelination, axonal injury, and neuronal loss. Our laboratory has developed a diffusion basis spectrum imaging (DBSI) technique to address the shortcomings of MRI-based MS. Primary DBSI metrics have been demonstrated to be associated with MS pathologies in animal models and human tissue. We propose that profiles of multiple DBSI metrics can identify important patterns within MS lesions and normal appearing white matter. Here we report that Diffusion Histology Imaging (DHI), an improved approach that combines a deep neural network (DNN) algorithm with DBSI-derived diffusion metrics, accurately detected and classified various MS lesion subtypes.
|
0041.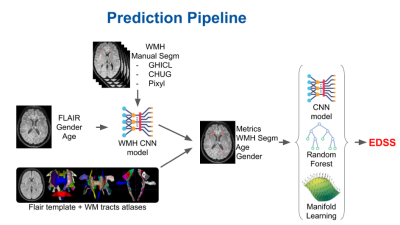 |
Breaking the clinico-radiological paradox in multiple sclerosis using machine learning
Arnaud Attyé1,2, Stenzel Cackowski3, Alan Tucholka4, Pauline Roca4, Pascal Rubini4, Sebastien Verclytte5, Lucie Colas5, Juliette Ding5, Jean-François Budzik5, Felix Renard6, Emmanuel L Barbier3, Romain Casey7,8,9,10, Sandra Vukusic7,8,
and François Cotton7,11
1Grenoble alpes university, Grenoble, France, 2Sydney Imaging Lab, Sydney university, Sydney, Australia, 3Univ. Grenoble Alpes, Inserm, U1216, Grenoble Institute Neurosciences, Grenoble, France, 4Pixyl Medical, Grenoble, France, 5Lille Catholic University, Lille, France, 6Laboratoire d'informatique de Grenoble, Grenoble, France, 7Claude Bernard Lyon 1 University, Lyon, France, 8Lyon University Hospital, Lyon, France, 9Observatoire Français de la Sclérose en Plaques, INSERM 1028 et CNRS UMR 5292, Lyon, France, 10EUGENE DEVIC EDMUS Foundation against multiple sclerosis, Lyon, France, 11CREATIS, CNRS UMR 5220 - INSERM U1206, Lyon, France
MRI is central to the study of white matter lesions in multiple sclerosis (MS). To date, the distribution of MS lesions, as evaluated on FLAIR imaging, has not been linked to patients’ disability prediction. Based on an international data challenge with 1500 MS patients and ground truth 2-year Expanded Disability Status Scale (EDSS), we have proposed an adaptive machine learning framework to predict the clinical disability. Here, we report the encouraging finding that our algorithm predicts the 2-year EDSS score with an accuracy estimated to 81%, only based on a single initial FLAIR sequence, added to sex and gender information.
|
|
0042.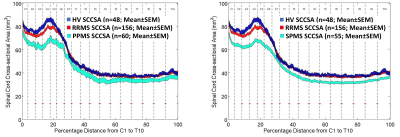 |
Cervical- and Thoracic-Cord Atrophy Correlates with Clinical Disability Scores in Various Multiple Sclerosis Phenotypes
Govind Nair1, Shila Azodi1, Tsemacha Dubuche1, Yair Mina1, Ikesinachi Osuorah1, Joan Ohayon1, Tianxia Wu1, Daniel S Reich1, and Steve Jacobson1
1National Institutes of Health, Bethesda, MD, United States
We sought to better understand the relationship between atrophy along the entire spinal cord and disease burden in multiple sclerosis using MRI. Towards this, we analyzed spinal cord cross-sectional area in 48 healthy control and 250+ subjects clinically diagnosed with various phenotypes of multiple sclerosis. Our results show cervical cord atrophy early in the onset of the disease, which correlated with clinical measures of disease severity. However, these correlations were reduced as the disease progressed. Such studies may help in better understanding of disease progression and can play a role as an imaging marker in clinical trials.
|
|
0043.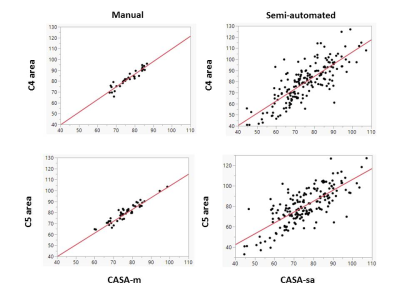 |
C5 level area can replace whole cervical spinal cord area measurements in multiple sclerosis as a practical biomarker of progression
Burcu Zeydan1,2, Selen Ucem2,3, Tsemacha Dubuche4, Shila Azodi4, Govind Bhagavatheeshwaran4,5, Jan-Mendelt Tillema 2, John Port1, Daniel Reich5, Steven Jacobson4, Kejal Kantarci1, and Orhun H. Kantarci2
1Radiology, Mayo Clinic, Rochester, MN, United States, 2Neurology, Mayo Clinic, Rochester, MN, United States, 3Marmara University School of Medicine, Istanbul, Turkey, 4Viral Immunology Section, Neuroimmunology and Neurovirology Division, National Institute of Neurological Disorders and Stroke, Bethesda, MD, United States, 5Translational Neuroradiology Section, Neuroimmunology and Neurovirology Division, National Institute of Neurological Disorders and Stroke, Bethesda, MD, United States
Subclinical progression reflecting neurodegeneration can be measured and followed through spinal cord volume monitoring in multiple sclerosis (MS). The increased atrophy is reflected more prominently in the caudal cervical spinal cord segment. In this study, we identified the C5 level area measurement which can reflect whole cervical spinal cord area in patients with MS using both semi-automated and manual measurements. We propose that the C5 level area measurement can replace whole cervical spinal cord area measurement in MS as a more practical biomarker of progression.
|
|
 |
0044.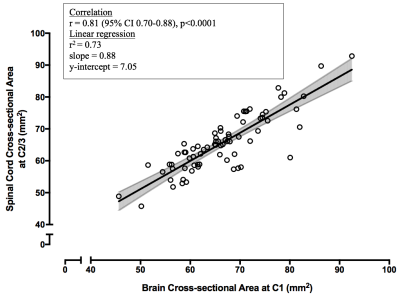 |
Accurate cervical spinal cord area measurements can be extracted from brain images
Kamyar Taheri1, Irene M. Vavasour1, Shawna Abel1, Lisa Eunyoung Lee1, Poljanka Johnson1, Stephen Ristow1, Roger Tam1, Cornelia Laule1, Nathalie Ackermans1, Alice J. Schabas1, Jillian Chan1, Ana-Luiza Sayao1, Virginia Devonshire1,
Robert Carruthers1, Anthony Traboulsee1, Shannon H. Kolind1, and Adam V. Dvorak1
1University of British Columbia, Vancouver, BC, Canada Multiple Sclerosis (MS) is a demyelinating disease of the central nervous system, with MRI routinely performed for brain but often neglected in spinal cord. When cord imaging IS performed, atrophy is usually assessed at the C2/3 segment. We aimed to validate cord cross-sectional-area (CSA) measurements using T1-weighted whole-brain images. In controls, strong correlations were seen between C1 CSA from cord and brain images, and between C1 and C2/3 CSA from cord images. In MS, C1 CSA from brain images and C2/3 CSA from cord images correlated. We showed that metrics obtained from brain images could provide relevant cord atrophy measures. |

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top