Scientific Session
Machine Learning and Tissue Characterisation in CMR
Session Topic: Machine Learning and Tissue Characterisation in CMR
Session Sub-Topic: CMR Tissue Characterisation
Oral
Cardiovascular
| Wednesday Parallel 1 Live Q&A | Wednesday, 12 August 2020, 14:30 - 15:15 UTC | Moderators: Walter Witschey |
Session Number: O-19
 |
0784.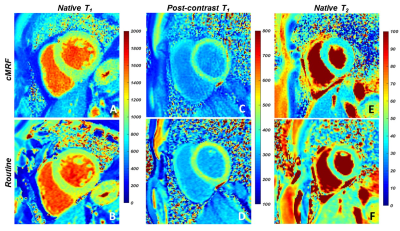 |
Cardiac MR fingerprinting with a short acquisition window in healthy volunteers and 62 consecutive patients referred for clinical CMR
Simone Rumac1, Anna Giulia Pavon2, Jesse Hamilton3, David Rodrigues1, Nicole Seiberlich3, Juerg Schwitter2, and Ruud B. van Heeswijk1
1Department of Radiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 2Cardiology Service, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 3Department of Radiology, University of Michigan, Ann Arbor, MI, United States
Cardiac magnetic resonance fingerprinting (cMRF) can be used to simultaneously acquire myocardial T1 and T2 maps in a single breath-hold. However, the common 250 ms acquisition window of cMRF might leave it vulnerable to motion artefacts. The goal of this study was therefore to compare the performance of cMRF with a short acquisition window (150ms) and low-rank reconstruction to that of routine cardiac parametric mapping techniques. In 7 healthy volunteers, and 62 cardiac patients, cMRF resulted in similar native relaxation times, but slightly different post-contrast T1 and ECV values compared to routine techniques.
|
 |
0785.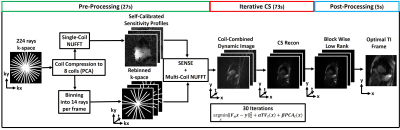 |
16-fold accelerated, single-shot late gadolinium enhancement CMR using GRASP for multi-TI reconstruction
Daming Shen1,2, Kyungpyo Hong3, Bradley D Allen2, Daniel C Lee4, and Daniel Kim1,2
1Biomedical Engineering, Northwestern University, Evanston, IL, United States, 2Radiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 3Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Division of Cardiology, Internal Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
Late gadolinium enhanced (LGE) CMR is the gold standard test for assessment of myocardial scarring. There is unmet need for high resolution, free-breathing LGE CMR for patients with arrhythmia and/or dyspnea. The purpose of this study was to develop and clinically evaluate a high resolution, free-breathing LGE CMR sequence combined with radial k-space sampling and compressed sensing (CS), which enables image contrast optimization without a TI scout.
|
 |
0786.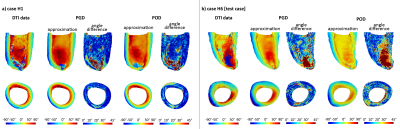 |
3D sub-millimeter personalized estimation of cardiomyocyte orientation using dimensionality reduction
Johanna Stimm1, Stefano Buoso1, Martin Genet2,3,4, Sebastian Kozerke1, and Christian T Stoeck1
1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland, 2Laboratoire de Mécanique des Solides, École Polytechnique, Paris, France, 3C.N.R.S./Université Paris-Saclay, Palaiseau, France, 4M3DISIM team, Inria / Université Paris-Saclay, Palaiseau, France
We propose a parametric low-rank representation of major characteristics of cardiomyocyte orientation in a shape-adapted coordinate system from 3D high-resolution ex-vivo cDTI data by exploiting structural similarity across hearts. We compare two dimensionality reduction methods, namely Proper Orthogonal Decomposition and Proper Generalized Decomposition. These low-order descriptions can be fit to sparse, noisy or low-resolution target data. Transferring high-resolution microstructural information with this parametric representation shows potential for in-vivo denoising and 3D extrapolation.
|
 |
0787.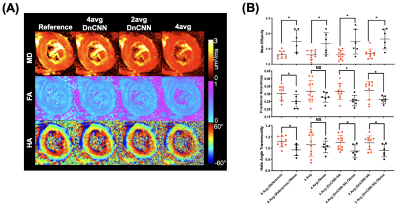 |
Accelerated In Vivo Cardiac Diffusion Tensor MRI with Residual Deep Learning based Denoising in Lean and Obese Subjects
Kellie Phipps1, Robert Eder1, Sam Allen Michelhaugh2, Aferdita Spahillari2, Maaike van den Boomen1,3,4, Joan Kim1, Shestruma Parajuli1, Timothy G Reese3,5, Choukri Mekkaoui3,5, David Sosnovik1,3,6, Denise Gee7,8, Ravi Shah1,6, and Christopher Nguyen1,3,6
1Cardiovascular Research Center, Massachusetts General Hospital, Charlestown, MA, United States, 2Cardiology Division, Massachusetts General Hospital, Charlestown, MA, United States, 3Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Radiology, University Medical Center Groningen, Groningen, Netherlands, 5Department of Radiology, Harvard Medical School, Boston, MA, United States, 6Department of Medicine, Harvard Medical School, Boston, MA, United States, 7Weight Center, Massachusetts General Hospital, Boston, MA, United States, 8Department of Surgery, Harvard Medical School, Boston, MA, United States
In vivo cardiac DT-MRI allows for imaging of the underlying myocardial fiber orientations but is hindered by clinically infeasible scan times. We developed and tested a residual deep learning denoising algorithm, DnCNN-54, on cardiac DT-MRI scans with fewer averages (4, 2, and 1) than the conventional 8-average 30 minute scan. We demonstrated a 2-fold acceleration can be achieved after DnCNN-54 is applied to 4 average dataset compared with the reference 8-average scan that preserves signal to noise ratio and cardiac DT-MRI parameter quantification. This 2-fold acceleration via DnCNN-54 denoising also maintained cardiac DT-MRI mean differences between obese and lean subjects.
|
 |
0788.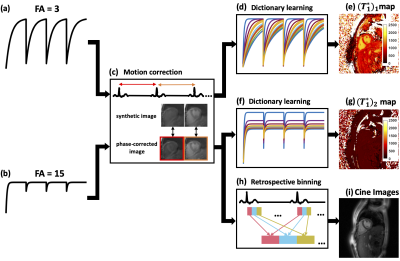 |
Free-breathing continuous cine and T1 mapping acquisition using a motion-corrected dual flip angle inversion-recovery spiral technique at 3 T
Ruixi Zhou1, Daniel S. Weller2, Yang Yang3, Junyu Wang1, John P. Mugler4, and Michael Salerno5
1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Electrical and Computer Engineering, University of Virginia, Charlottesville, VA, United States, 3Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Radiology, Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 5Cardiology, Radiology, Biomedical Engineering, University of Virginia, Charlottesville, VA, United States
We propose a technique to obtain cine images and accurate B1-corrected T1 maps in a single free-breathing continuous Look-Locker inversion-recovery acquisition modified to use two excitation flip angles. Data are acquired using a single spiral interleaf, rotated by the golden-angle in time, with an inversion RF pulse applied every four seconds. Cine images are reconstructed from the steady state portion of the signal, while T1 mapping fits the model using maps with two flip angles. This strategy provides cine images and T1 maps, as well as a flip angle scale factor map, in a single free-breathing continuous acquisition.
|
 |
0789.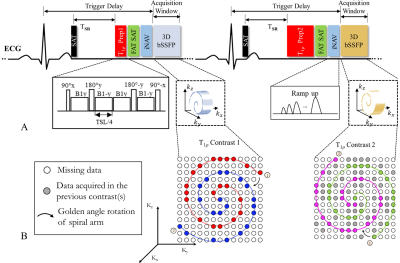 |
Respiratory Motion-compensated High-resolution 3D Whole-heart T1ρ Mapping
Haikun Qi1, Aurelien Bustin1, Thomas Kuestner1, Reza Hajhosseiny1, Gastao Cruz1, Karl Kunze1,2, Radhouene Neji1,2, René Botnar1, and Claudia Prieto1
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Cardiac T1ρ mapping has shown promising results for detecting ischemic cardiomyopathy without the need of exogenous contrast agents. Current 2D myocardial T1ρ mapping requires multiple breath-holds and provides limited coverage of the heart. In this study, we proposed a free-breathing 3D T1ρ mapping technique featuring whole heart coverage, near-isotropic spatial resolution (1.7×1.7×2mm3) and 100% respiratory acquisition efficiency. With the proposed technique, five T1ρ weightings were acquired in a clinically feasible scan time (~6 min), based on which 3D T1ρ maps were estimated. The accuracy and feasibility of the 3D technique was investigated in phantoms, healthy subjects and patient.
|
0790.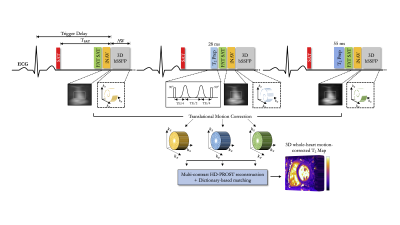 |
Reproducibility, Repeatability and Preliminary Clinical Results of Free-Breathing Isotropic 3D Whole-Heart T2 Mapping
Aurelien Bustin1, Alina Hua1, Giorgia Milotta1, Olivier Jaubert1, Reza Hajhosseiny1, Tevfik Ismail1, René Botnar1, and Claudia Prieto1
1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Myocardial T2 mapping has emerged as a promising tool for edema characterization and detection of myocardial inflammation. T2 mapping is conventionally performed under breath-hold by acquiring multiple T2-prepared images using 2D bSSFP. However, 2D imaging and breath-holding impedes high spatial resolution, limits whole-heart coverage and can be challenging in some patients. We developed a free-breathing 3D whole-heart T2 mapping framework that achieves high isotropic resolution in a clinically feasible scan time in healthy subjects. Here we sought to quantify the reproducibility and repeatability of this technique and assess its performance to detect myocardial inflammation in a clinical setting.
|
|
0791.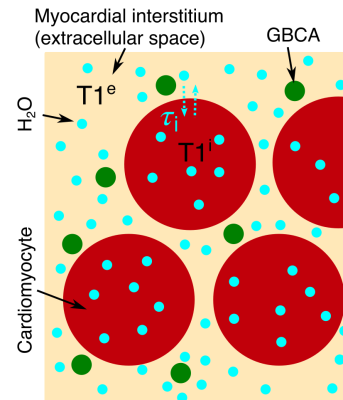 |
Quantifying the underestimation of myocardial extra cellular volume fraction measurements due to transcytolemmal water exchange
Andrew D Scott1,2, Peter D Gatehouse1,2, and David N Firmin1,2
1CMR Unit, The Royal Brompton Hospital, London, United Kingdom, 2National Heart and Lung Institute, Imperial College London, London, United Kingdom
MR based measures of myocardial extra cellular volume fraction (ECV) obtained from pre and post-contrast T1 mapping are frequently used in research studies. However, typically ECV calculations rely on rapid exchange of water molecules between the intra and extracellular space. We assess the validity of the shutter speed approximation of the full two-compartment model and use this model to assess the effect of limited water exchange rate between the cardiomyocytes and interstitial fluid. For typical conditions used in measuring ECV, we demonstrate an underestimation of ECV on a similar magnitude to the changes attributed to disease in some studies.
|
|
0792.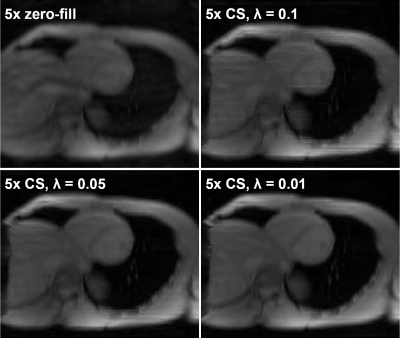 |
Effects of Accelerated Acquisition of Myocardial Creatine CEST MRI in the Healthy Human Heart at 3T
Kevin Godines1, Wissam AlGhuraibawi1, Bonnie Lam1, and Moriel Vandsburger1
1Bioengineering, University of California Berkeley, Berkeley, CA, United States
CEST-MRI is emerging as a powerful modality for molecular imaging of cardiac metabolites and fibrosis. In order to derive all contrasts (amide proton transfer: APT, creatine, and magnetization transfer: MT) a substantial number of differently CEST-weighted images must be acquired. Application of compressed sensing for cardiac CEST enables a 5x acceleration of acquisition with preserved accuracy.
|
|
0793.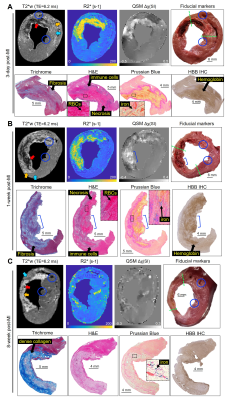 |
Magnetic susceptibility, R2* and iron evolve during reperfusion injury wound healing
Brianna F. Moon1, Srikant Kamesh Iyer2, Eileen Hwuang1, Nicholas J. Josselyn2, James J. Pilla2, Joseph H. Gorman III3, Robert C. Gorman3, Cory Tschabrunn4, Samuel J. Keeney3, Estibaliz Castillero5, Giovanni Ferrari5, Steffen Jockusch6, Haochang Shou7,
Elizabeth M. Higbee-Dempsey8, Andrew Tsourkas1, Victor A. Ferrari4, Yuchi Han4, Harold I. Litt2, and Walter R. Witschey2
1Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 4Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 5Surgery, Columbia University Irving Medical Center, New York City, NY, United States, 6Chemistry, Columbia University, New York City, NY, United States, 7Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 8Biochemistry and Molecular Biophysics Graduate Group, Perelman School of medicine, University of Pennsylvania, Philadelphia, PA, United States
There are multiple forms of iron including protein bound and labile iron found in reperfusion injury of acute myocardial infarction (MI). This study investigated iron accumulation, molecular form of iron, and cellular response to reperfusion injury with respect to the duration of wound-healing, in a large animal model. We demonstrate with magnetic susceptibility and R2* imaging biomarkers, there is a significant increase in infarct iron content in acute reperfusion injury that dissipates by 8-week post-MI and validate these findings with histology, iron concentration, and mRNA expression.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top