Scientific Session
Advances in Quantitative MRI
Session Topic: Advances in Quantitative MRI
Session Sub-Topic: Advances in MR Fingerprinting
Oral
Acquisition, Reconstruction & Analysis
| Wednesday Parallel 5 Live Q&A | Wednesday, 12 August 2020, 14:30 - 15:15 UTC | Moderators: Guido Buonincontri & Debra McGivney |
Session Number: O-59
 |
0867.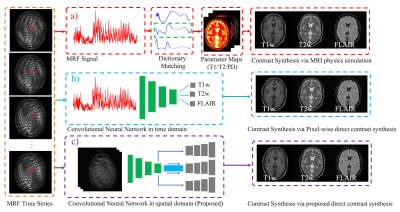 |
High Fidelity Direct-Contrast Synthesis from Magnetic Resonance Fingerprinting in Diagnostic Imaging
Ke Wang1, Mariya Doneva2, Thomas Amthor2, Vera C. Keil3, Ekin Karasan1, Fei Tan4, Jonathan I. Tamir1,5, Stella X. Yu1,6, and Michael Lustig1
1Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States, 2Philips Research, Hamburg, Germany, 3Universitätsklinikum Bonn, Bonn, Germany, 4Bioengineering, UC Berkeley-UCSF, San Francisco, CA, United States, 5Electrical and Computer Engineering, The University of Texas at Austin, Austin, TX, United States, 6International Computer Science Institute, University of California, Berkeley, Berkeley, CA, United States
MR Fingerprinting is an emerging attractive candidate for multi-contrast imaging since it quickly generates reliable tissue parameter maps. However, contrast-weighted images generated from parameter maps often exhibit artifacts due to model and acquisition imperfections. Instead of direct modeling, we propose a supervised method to learn the mapping from MRF data directly to synthesized contrast-weighted images, i.e., direct contrast synthesis (DCS). In-vivo experiments on both volunteers and patients show substantial improvements of our proposed method over previous DCS method and methods that derive synthetic images from parameter maps.
|
0868.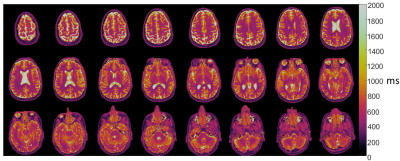 |
Feasibility of MR fingerprinting using a high-performance 0.55T MRI system
Adrienne E. Campbell-Washburn1, Yun Jiang2, Gregor Körzdörfer3,4, Mathias Nittka3, and Mark A. Griswold2
1National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Department of Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
We assess the feasibility of T1 and T2 mapping with MR fingerprinting implemented on a high-performance 0.55T system that combines contemporary hardware and imaging methods with a lower magnetic field strength. Quantitative values correlated closely to spin-echo measurements in the NIST phantom. Brain MRF was evaluated in 12 healthy volunteers and liver MRF was evaluated in one volunteer as a proof-of-concept. At 0.55T, T1 was 539ms (white matter) and 660ms (gray matter), and T2 was 64ms (white matter) and 76ms (gray matter). The combination of MRI fingerprinting and low-field MRI systems provides an opportunity for rapid, low-cost, quantitative imaging.
|
|
 |
0869.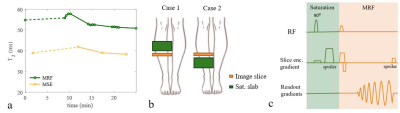 |
An attempt to understand why we measure longer relaxation times in quantitative muscle MRI using MRF than using conventional methods
Kirsten Koolstra1, Andrew Webb1, and Peter Börnert1,2
1Leiden University Medical Center, Leiden, Netherlands, 2Philips Research Hamburg, Hamburg, Germany
Fast relaxation time quantification is important in dynamic muscle studies and can be achieved using Magnetic Resonance Fingerprinting (MRF). The T2 values in muscle measured with MRF are consistently higher than those measured with the conventionally used multi-echo turbo-spin-echo (MSE) method, while T1 values are closer to reference measurements. We hypothesize that this increase can in part be attributed to an increased sensitivity of MRF to flow compared to MSE. In this work we test the sensitivity of MRF to flow in muscle by saturating a slab at different distances above the imaging slice for variable suppression of inflowing spins.
|
 |
0870.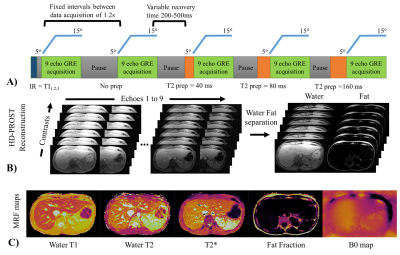 |
Liver Dixon MR Fingerprinting: T1, T2, T2* and fat fraction tissue characterization
Olivier Jaubert1, Cristobal Arrieta2,3, Gastao Cruz1, Aurelien Bustin1, Torben Schneider4, Georgios Georgiopoulos1, Pier-Giorgio Masci1, Carlos Sing-Long3,5,6, Rene Michael Botnar1, and Claudia Prieto1
1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Biomedical Imaging Center, Pontificia Universidad Católica de Chile, Santiago, Chile, 3Millennium Nucleus for Cardiovascular Magnetic Resonance, Santiago, Chile, 4Philips Healthcare, London, United Kingdom, 5Instituto de Ingeniería Matemática y Computacional, Pontificia Universidad Católica de Chile, Santiago, Chile, 6Millennium Nucleus Center for the Discovery of Structures in Complex Data, Chile, Santiago, Chile
Quantitative T1, T2, T2* and fat fraction (FF) maps are promising imaging biomarkers for the assessment of liver disease. Magnetic Resonance Fingerprinting has been recently proposed for fast T1, T2 and M0 mapping of the liver, however in the presence of high iron or fat concentrations corrections using separately acquired T2* and FF maps are needed. Here we propose a novel approach which enables simultaneous liver T1, T2, T2* and FF maps from a single ~15s breath-hold scan. The proposed approach was evaluated on phantoms, 8 healthy subjects and 2 patients in comparison to
conventional mapping techniques.
|
0871.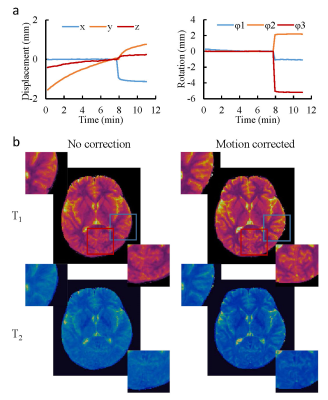 |
Improving motion robustness of 3D MR Fingerprinting using fat navigator
Yong Chen1, Xiaopeng Zong2, Dan Ma1, Weili Lin2, and Mark Griswold1
1Radiology, Case Western Reserve University, Cleveland, OH, United States, 2Radiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
In this study, we developed a 3D MRF method in combination with fat navigator to improve its motion sensitivity for neuroimaging. A rapid fat navigator sampling was achieved at 3T by using the stack-of-spirals acquisition and non-Cartesian spiral GRAPPA. The improvement in motion robustness was achieved without increasing the scan time for quantitative tissue mapping. Our preliminary results demonstrate that 1) the added fat navigator sampling does not influence the accuracy of T1 and T2 quantification, and 2) the motion robustness for quantitative tissue mapping using MRF was largely improved with the proposed method.
|
|
 |
0872.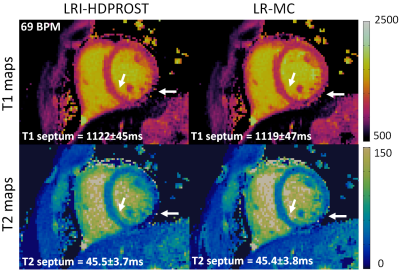 |
Generalised Low-Rank Non-rigid Motion Corrected reconstruction for 2D Cardiac MRF
Gastao Cruz1, Haikun Qi1, Olivier Jaubert1, Aurelien Bustin1, Thomas Kuestner1, Torben Schneider2, René M. Botnar1, and Claudia Prieto1
1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Philips Healthcare, Guildford, United Kingdom
Cardiac Magnetic Resonance Fingerprinting (cMRF) has been proposed for simultaneous myocardial T1 and T2 mapping. This approach uses ECG-triggering to synchronize data acquisition to a small mid-diastolic window, reducing cardiac motion artefacts but also limiting the amount of acquired data per heartbeat. This low scan efficiency can limit the spatial resolution achievable in a breath-held scan. Here we introduce a novel approach for contrast-resolved motion-corrected reconstruction, that combines the generalized matrix description formulism for non-rigid motion correction with low-rank compression of temporally varying contrast. This approach enables longer acquisition windows and higher scan efficiency in cMRF, correcting for cardiac motion.
|
 |
0873.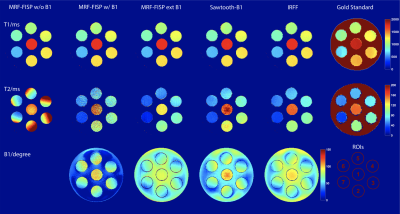 |
Evaluation of transmit sensitivity (B1+) encoding in MR fingerprinting at 7T
Ding Xia1,2, Zidan Yu1,2,3, Riccardo Lattanzi1,2,3, and Martijn A Cloos1,2
1Center for Advanced Imaging Innovation and Research, Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States, 3Sackler Institute of Graduate Biomedical Sciences, New York University School of Medicine, New York, NY, United States
We evaluated the ability of three reported MR fingerprinting methods to mitigate the effect of B1+ inhomogeneity at 7T. Results from each method were compared with gold standard results. All methods provided relatively accurate T1 quantification. We show that T2 cannot be accurately quantified at 7T without accounting for B1+ in the MR fingerprinting dictionary. The Inversion-Recovery-FISP-FLASH (IRFF) method provided the most accurate T2 values. We conclude that the use of both FISP and FLASH segments best encodes B1+ into the fingerprint.
|
 |
0874.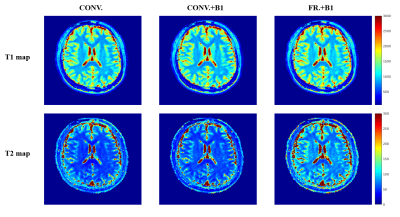 |
Experimental Comparisons of MRF-FISP Using Optimized Fractional Dictionary and B1 Correction
Lixian Zou1,2, Huihui Ye3, Shi Su1, Haifeng Wang1, Dong Liang1,4, Xin Liu1, and Hairong Zheng1
1Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences, Shenzhen, China, 2Shenzhen College of Advanced Technology, University of Chinese Academy of Sciences, Shenzhen, China, 3State Key Laboratory of Modern Optical Instrumentation, College of Optical Science and Engineering, Zhejiang University, Zhejiang, China, 4Research Center for Medical AI, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
MRF is a time-efficient technique to simultaneously measure of multiple parameters through pattern recognition. The completeness of dictionary to describe the signal evolution process in NMR system is very important to acquire the accurate T1 and T2 values. A new dictionary, generated by using fractional Bloch equations and B1 correction, is proposed to improve the MRF-FISP accuracy. In this work, we compared the accuracy of relaxation values with three dictionary models through phantom and in-vivo experiments. Results illustrated that dictionary generated through fractional Bloch equation with B1 correction is the best to approach T1 and T2 standards.
|
0875.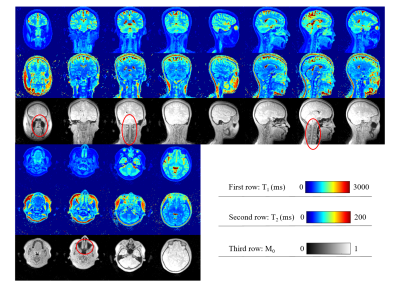 |
3D UTE-MRF for multiple parametric maps with sub-millimeter isotropic resolution using multi-dimensional golden-angle radial trajectory
Qing Li1,2, Xiaozhi Cao1, Huihui Ye1,3, Zihan Zhou1, Hongjian He1, and Jianhui Zhong1
1Center for Brain Imaging Science and Technology, Key Laboratory for Biomedical Engineering of Ministry of Education, College of Biomedical Engineering and Instrumental Science, Zhejiang University, Hangzhou, China, 2Siemens Healthcare Ltd., Shanghai, China, 3State Key Laboratory of Modern Optical Instrumentation, College of Optical Science and Engineering, Zhejiang University, Hangzhou, China In this study, we used a 3D ultrashort-echo-time MR Fingerprinting (UTE-MRF) method to generate distortion-free quantitative T1, T2, and proton density maps with an isotropic resolution of 0.8 x 0.8 x 0.8 mm3. |
|
0876.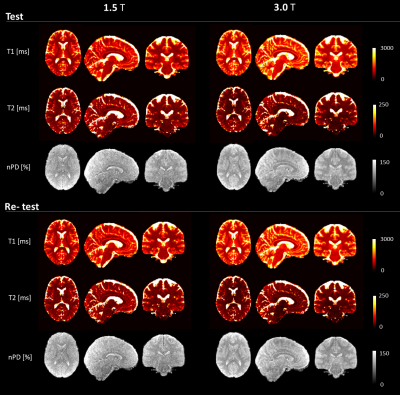 |
Three-dimensional MRF obtains highly repeatable and reproducible multi-parametric estimations in the healthy human brain at 1.5T and 3T
Guido Buonincontri1,2, Jan W Kurzawski2,3, Joshua Kaggie4, Tomasz Matys4, Ferdia Gallagher4, Matteo Cencini2,5, Graziella Donatelli2,6, Paolo Cecchi6, Mirco Cosottini2,6,7, Nicola Martini8, Francesca Frijia9, Domenico Montanaro9, Pedro A Gómez2,10,
Rolf F Schulte11, Alessandra Retico3, and Michela Tosetti1,2
1IRCCS Stella Maris, Pisa, Italy, 2Imago7 Foundation, Pisa, Italy, 3Istituto Nazionale di Fisica Nucleare, Pisa, Italy, 4Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 5University of Pisa, Department of Physics, Pisa, Italy, 6U.O. Neuroradiologia, Azienda Ospedaliera Universitaria Pisana (AOUP), Pisa, Italy, 7University of Pisa, Department of Translational Research and New Technologies in Medicine and Surgery, Pisa, Italy, 8Fondazione Toscana Gabriele Monasterio, Pisa, Italy, 9U.O.C. Risonanza Magnetica Specialistica e Neuroradiologia, Fondazione CNR/Regione Toscana G. Monasterio, Pisa, Italy, 10Technical University of Munich, Munich, Germany, 11GE Healthcare, Munich, Germany
Three-dimensional magnetic resonance fingerprinting with spiral projection k-space trajectory offers fully-quantitative estimations at a high spatial resolution. To assess the repeatability and reproducibility of the estimations, we acquired test/re-test data in the human brain at 1.5T and 3.0T in a travelling head study involving a total of 12 subjects and 8 different MR scanners. Our approach estimated voxel-wise performance in the CNS: variability was assessed using coefficients-of-variation, bias using a GLM analysis. Solid matter repeatability CVs were under 2% for nPD/T1, and 5% for T2, while reproducibility biases were under 10% in solid matter compartments for T1/T2.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top