Scientific Session
Diffusion Acquisition and Reconstruction
Session Topic: Diffusion Acquisition and Reconstruction
Session Sub-Topic: Diffusion: Reconstruction & Artefact Correction
Oral
Diffusion
| Wednesday Parallel 4 Live Q&A | Wednesday, 12 August 2020, 15:15 - 16:00 UTC | Moderators: Wu Dan & HAO HUANG |
Session Number: O-71
 |
0978.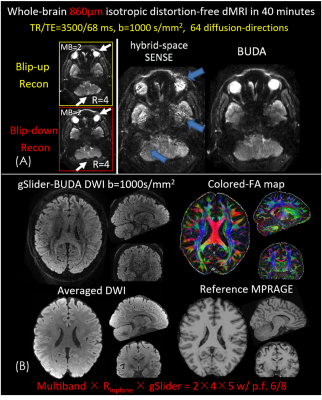 |
Distortion-free, submillimeter-isotropic-resolution diffusion MRI with gSlider BUDA-EPI and multi-coil dynamic B0 shimming
Congyu Liao1, Berkin Bilgic1, Qiyuan Tian1, Jason Stockmann1, Qiuyun Fan1, Siddharth Srinivasan Iyer1,2, Fuyixue Wang1,3, Chanon Ngamsombat1,4, Xiaozhi Cao1, Mary Kate Manhard1, Susie Y. Huang1, Lawrence L. Wald1, and Kawin Setsompop1
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 2Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Department of Radiology, Faculty of Medicine, Siriraj Hospital, Mahidol University, Thailand
Diffusion magnetic resonance imaging (dMRI) is a highly sensitive imaging modality, but is limited in spatial resolution and signal-to-noise ratio (SNR). In this work, we combine an SNR-efficient acquisition and model-based reconstruction strategies with newly-available hardware instrumentation to achieve distortion-free in-vivo dMRI at 600-860 µm isotropic voxel size with high fidelity and sensitivity on a clinical 3T scanner. At this resolution, it is possible to accurately probe the microstructure of different cortical layers in the human brain.
|
 |
0979.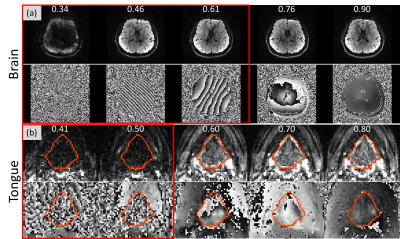 |
Prospective Motion Detection and Re-acquisition in Diffusion MRI using Phase image-based Method (PITA-MDD)
Xiao Liang1, Pan Su2, Sunil G. Patil2, Nahla M.H. Elsaid3, Steve Roys1, Maureen L. Stone4, Rao P. Gullapalli1, Jerry L. Prince5,6, and Jiachen Zhuo1
1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Siemens Medical Solutions USA Inc, Malvern, PA, United States, 3Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States, 4Department of Neural and Pain Sciences and Department of Orthodontics, University of Maryland School of Dentistry, Baltimore, MD, United States, 5Deptartment of Electrical and Computer Engineering, Johns Hopkins University, Baltimore, MD, United States, 6Department of Computer Science, Johns Hopkins University, Baltimore, MD, United States
We have applied phase image-based motion detection (PITA-MDD) in real-time prospective motion detection and re-acquisition. During image reconstruction, PITA-MDD motion detection is performed on each slice. A diffusion-weighted volume will be re-acquired if number of motion slices exceeds the pre-set threshold. dMRI data were acquired on a volunteer using a prospective PITA-MDD sequence for the brain and the tongue. The detected motion corrupted data were consistent with subject’s motion. Denser tongue muscle fibers were visible after replacing motion volumes with re-acquired volumes. Prospective PITA-MDD motion detection and re-acquisition has improved dMRI acquisition, especially in challenging areas, such as the tongue.
|
0980.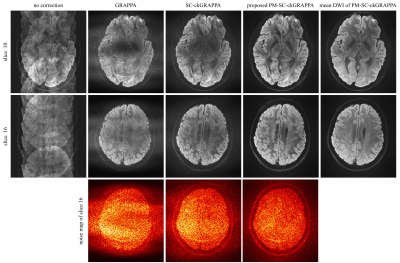 |
Phase-matched Self-calibrated K-space Phase Correction Method for Multi-shot Diffusion Imaging
Zhe Zhang1, Xiaodong Ma2, Lanxin Ji1, Jing Jing1, Wanlin Zhu1, Zhangxuan Hu3, Yishi Wang4, Hua Guo3, and Yongjun Wang1,5
1China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijng, China, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 4Philips Healthcare, Beijing, China, 5Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Multi-shot acquisition enables high-resolution diffusion imaging, but the artifacts caused by shot-to-shot phase variation must be corrected. Self-calibrated multi-shot DWI methods utilize parallel imaging reconstruction to solve the phase of each shot. Previously reported self-calibrated GRAPPA with a compact kernel (SC-ckGRAPPA) method is compromised by the high reduction factor when recovering the navigator information. In this work, PM-SC-ckGRAPPA was introduced with the phase-matched reconstruction, and evaluated via in-vivo experiment. Results show that PM-SC-ckGRAPPA provides improved reconstruction compared with conventional approaches, and PM-SC-ckGRAPPA can be a potential tool for high-resolution diffusion imaging.
|
|
0981.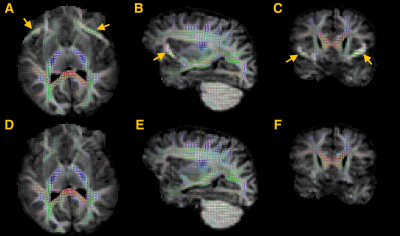 |
Fat-shift suppression in diffusion MRI using rotating phase encoding and localised outlier weighting
Daan Christiaens1,2, Lucilio Cordero-Grande1,3, Jana Hutter3,4, Anthony N Price3,4, Jonathan O'Muircheartaigh1, Katy Vecchiato1, Joseph V Hajnal1,3, and J-Donald Tournier1,3
1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Electrical Engineering (ESAT/PSI), KU Leuven, Leuven, Belgium, 3Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 4Centre for the Developing Brain, King's College London, London, United Kingdom
Diffusion MRI is prone to fat-shift artefacts, especially in accelerated diffusion MRI with higher b-values. Building on the property that the fat signal localisation depends on the phase encoding direction, we propose to suppress fat-shift artefacts in post-processing using localised outlier rejection across 4 different phase encoding directions. To this end, we extend a retrospective diffusion MRI motion correction framework with local outlier weights, defined as a voxel-wise measure of the MR reconstruction residuals. Comparative results in a pediatric brain imaging cohort show that the proposed method reduces fat-shift artefacts in the parenchyma without affecting the reconstruction in uncorrupted regions.
|
|
0982.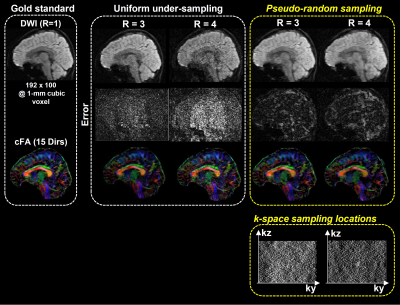 |
High-Resolution 3D Multi-shot Diffusion-Weighted Imaging with Pseudo-Random Sampling and Compressed Sensing
Hing-Chiu Chang1 and Xiaoxi Liu1,2
1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
3D multi-shot diffusion-weighted imaging (msDWI) with multi-slab acquisition can achieve high-resolution diffusion-tensor imaging (DTI), but additional correction is required to eliminate slab boundary artifact associated with multi-slab acquisition. A proposed 3D-MUSER technique can improve the feasible slab thickness by enabling 3D phase correction with a 3D single-shot navigator, thereby making single-slab 3D DTI feasible. However, the relatively long scantime can limit the applications of 3D DTI in neuroscience research, despite high spatial resolution attainable. In this study, we proposed a potential strategy to develop a 3D DTI technique capable of high scan acceleration.
|
|
0983.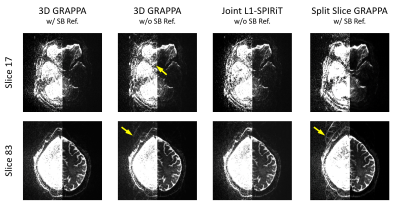 |
Coil-joint-split N/2 Ghost Correction and Joint L1-SPIRiT for SMS-EPI Reconstruction: Demonstration Using 7T HCP-style Diffusion Acquisition
Ziyi Pan1, Hua Guo1, Erpeng Dai2, Edward J. Auerbach3, Kamil Ugurbil3, and Xiaoping Wu3
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Department of Radiology, Stanford University, Stanford, CA, United States, 3Center for Magnetic Resonance Research, Radiology, Medical School of the University of Minnesota, Minneapolis, MN, United States
Simultaneous Multislice (SMS) has become a major acceleration technique in Human Connectome Project (HCP) to acquire high-resolution diffusion and functional MRI. Conventional reconstruction for SMS-EPI includes using traditional Nyquist ghost correction and slice GRAPPA that usually requires single-band (SB) reference scans. In this work, we introducea a novel reference-less Nyquist ghost correction approach and a new joint L1-spirit reconstruction algorithm without the need of SB reference scans. We evaluated the performance of the proposed method by acquiring 7T HCP-style diffusion and show that the proposed method can effectively suppress the strong residual aliasing/ghosting as observed for when using conventional reconstruction.
|
|
 |
0984.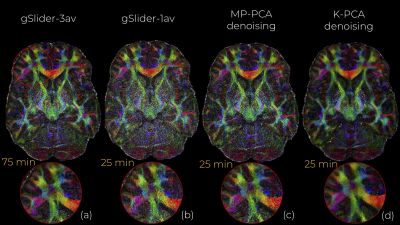 |
Structure preserving noise removal in Hilbert space from ultra-high resolution diffusion MRI data
Gabriel Ramos-Llordén1, Gonzalo Vegas-Sanchez-Ferrero1, Congyu Liao2, Carl-Fredrik Westin1, Kawin Setsompop2, and Yogesh Rathi1
1Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States, 2Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States
In-vivo submillimeter resolution diffusion MRI suffers from limited signal-to-noise ratio (SNR) due to the small voxel size. Denoising techniques can improve the SNR and facilitate further dMRI analysis. Among them, perhaps PCA-based (e.g, Marchenko-Pastur PCA) have shown the best performance. In this work, we introduce kernel PCA, a powerful nonlinear generalization of linear PCA to Hilbert spaces that is shown to suppress a substantial amount of noise (which MP-PCA is incapable of) and still reliably preserve dMRI signal. We showcase K-PCA noise removal with 660 micrometer gSlider data, where we compared it qualitatively and qualitatively with MP-PCA.
|
0985.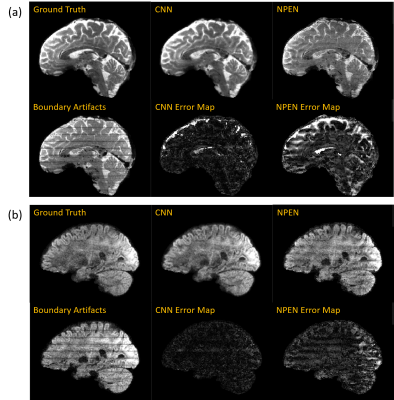 |
Rapid Boundary Artifacts Correction for Simultaneous Multi-slab (SMSlab) Acquisition Using Convolutional Network
Jieying Zhang1, Simin Liu1, Yuhsuan Wu1, and Hua Guo1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China
Simultaneous multi-slab (SMSlab) technique is a 3D acquisition method that can achieve optimal signal-to-noise ratio (SNR) efficiency for high-resolution diffusion-weighted imaging (DWI) or functional MRI (fMRI). However, boundary artifacts may restrain its application. Nonlinear inversion for slab profile encoding (NPEN) has been proposed for its correction, which needs long computation time. In this study, we propose to use a convolutional network for boundary artifacts correction. It can solve the problem in a short time and improve the signal-to-noise ratio (SNR), which is of great meaning for high-resolution whole-brain DWI and fMRI.
|
|
0986.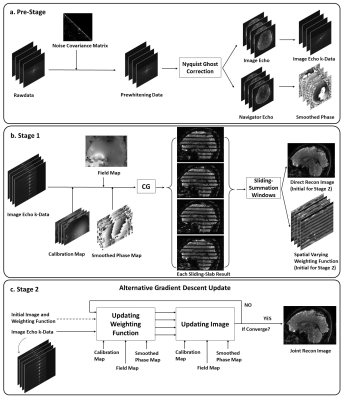 |
Sliding-Slab Profile Encoding (SLIPEN) for Eliminating Slab Boundary Artifact in Three-Dimensional Multi-slab Diffusion-Tensor Imaging
Xiaoxi Liu1,2, Di Cui1, Xucheng Zhu2, Edward S. Hui1,3, Queenie Chan4, Peder E.Z. Larson2, and Hing-Chiu Chang1
1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, China, 2Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 3The State Key Laboratory of Brain and Cognitive Sciences, Hong Kong, China, 4Philips Healthcare, Hong Kong, China
3D multi-slab diffusion-tensor imaging (DTI) can enable high-resolution DTI at submillimeter voxel size. However, the slab boundary artifact and distortion along slab direction can deteriorate the data quality of 3D DTI, thereby limiting its applications. In this work, we proposed a sliding-slab profile encoding (SLIPEN) method to acquire the 3D multi-slab DTI data with sliding-slab technique, and to reconstruct the data free from slab boundary artifact. In addition, off-resonance correction can be incorporated into SLIPEN for producing high-quality artifact-free 3D DTI data.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top