Scientific Session
Multimodal fMRI
Session Topic: Multimodal fMRI
Session Sub-Topic: fMRI in Animal Models
Oral
fMRI
| Thursday Parallel 4 Live Q&A | Thursday, 13 August 2020, 15:50 - 16:35 UTC | Moderators: Shella Keilholz |
Session Number: O-73
 |
1349.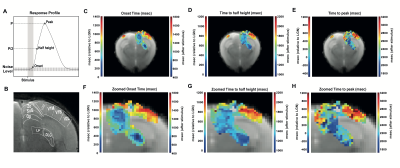 |
Ultrafast functional MRI signals reflect activation sequence in the mouse visual pathway
Rita Gil1, Francisca F. Fernandes1, and Noam Shemesh1
1Champalimaud Neuroscience Programme, Champalimaud Centre for the Unknown, Lisbon, Portugal
We investigated BOLD response profiles along the entire mouse visual pathway (with monocular stimulation) using an ultrafast fMRI acquisition with 50 ms temporal resolution and quantified onset, half-maximum and peak times. To achieve the spatial coverage with this temporal resolution, an oblique slice covering the entire visual pathway was tailored. The quantified onset times were the only parameter correlating with the visual pathway neural input order. Our findings highlight a potential importance for onset time quantification – requiring ultrafast fMRI acquisitions – as a signature capable of mapping the underlying activation sequence of events in distributed neural networks.
|
 |
1350.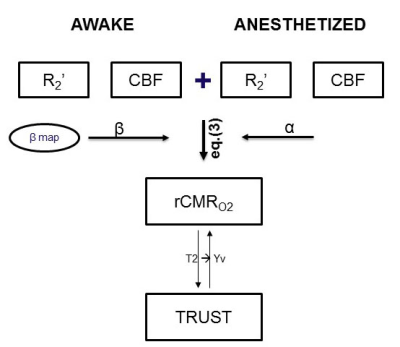 |
M-Mapping Method for Calibrated fMRI in Awake Versus Anesthetized Mice
Binshi Bo1, Mengyang Xu2, Garth Thompson2, and Zhifeng Liang1
1Institute of Neuroscinece, CAS Center for Excellence in Brain Sciences and Intelligence Technology, Key Laboratory of Primate Neurobiology, Chinese Academy of Sciences, Shanghai, China, 2iHuman Institute, ShanghaiTech University, Shanghai, China
In functional brain imaging, the BOLD signal represents a mixture of CBF, CBV and the CMRO2. “Calibrated fMRI” methods aim to measure CMRO2 through a metabolic model, but typically require administration of gases which is not possible in many clinical settings. Here, we developed a calibrated fMRI technique called “M-Mapping” which combines CBF and R2' maps together to calculate relative CMRO2 and showed awake mice has a greater value than anesthetized mice in a region specific manner. This method may have a great potential to compare brain metabolic activity across different resting activity levels for further clinical study.
|
 |
1351.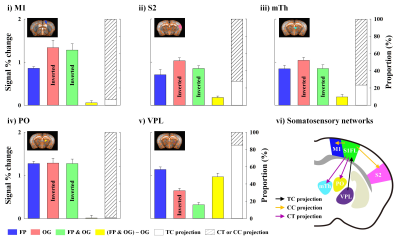 |
Functional dissection of somatosensory processing pathways in mice
Won Beom Jung1,2, Hyun Seok Moon1,2, Taeyi You1,2, Jung Mi Lee1, and Seong-Gi Kim1,2
1Cener for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon-si, Gyeonggi-do, Korea, Republic of, 2Department of Biomedical Engineering, Sungkyunkwan University, Suwon-si, Gyeonggi-do, Korea, Republic of
Somatosensory system is communicated by feedforward and feedback projection each other during functional processing. Somatosensory fMRI response is attributed to these inter-regional reciprocal projections. Therefore, the separation of functional pathways in fMRI data is important to interpret fMRI data in circuit level. Here, to dissect somatosensory fMRI response, we compared CBV-weighted fMRI obtained at 15.2T under three conditions: excitation by sensory stimulation, silencing of somatosensory cortex by optogenetic stimulation, and combined excitation and silencing.
|
 |
1352.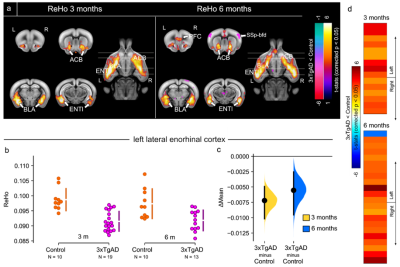 |
Brain-wide functional mapping of the entorhinal cortex in young 3xTg mouse model for Alzheimer’s disease
Francesca Mandino1,2, Ling Yun Yeow2, Chai Lean Teoh2, Chun-Yao Lee2, Renzhe Bi2, Hasan Mohammad2, Sejin Lee2, Han Gyu Bae2, Seung Hyun Baek2, Hanqing Jasinda Lee3, Kim Peng Mitchell Lai3, Sangyong Jung2, Fu Yu2, Malini
Olivo2, John Gigg1, and Joanes Grandjean4
1Faculty of biology, medicine and health, University of Manchester, manchester, United Kingdom, 2Singapore Bioimaging Consortium, A*STAR, Singapore, Singapore, Singapore, 3Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, 4Department of Radiology and Nuclear Medicine & Donders Institute for Brain, Cognition, and Behaviour, Donders Institute, Radboud University Medical Centre, Nijmegen, Netherlands
Alzheimer’s disease (AD) is characterised by progressive memory loss, neurodegeneration and brain atrophy. Intra- and inter-regional connectivity across the brain is affected in AD, probably due to the aberrant accumulation of toxicity. The entorhinal cortex is a key region involved in the early stages of AD. We report synaptic connectivity increase in the 3xTg mouse model, by means of electrophysiological recordings in AD-susceptible brain regions, following stimulation of the entorhinal cortex, in vivo. Further, we demonstrate loss of functional connectivity with resting-state fMRI in AD-vulnerable brain regions, which converts into increased response during optogenetics photostimulation of the entorhinal cortex.
|
 |
1353.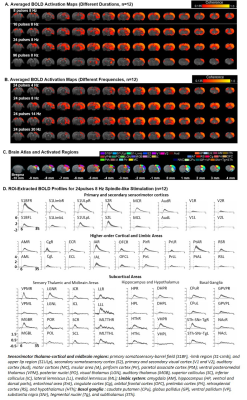 |
Functional MRI investigation of Optogenetically-evoked Spindle-like Neural Activity and Memory Consolidation
Xunda Wang1,2, Alex T. L. Leong1,2, Shawn Zheng kai Tan3, Teng Ma1,2, Pek-Lan Khong4, Lee-Wei Lim3, and Ed Xuekui Wu1,2,3,4
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, Hong Kong, 3School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong, 4Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong
Spindle is one of the most critical brain oscillatory activities that has been shown to mediate sensory transmission and memory consolidation. However, where and how spindle-related activities are distributed at the systems level and their brain-wide propagation targets remain elusive. In this study, we demonstrate the first integrative view of the causal recruitment of brain-wide networks by thalamo-cortically initiated spindle-related activities in a temporal-frequency specific manner and verified its role in facilitating memory consolidation.
|
 |
1354.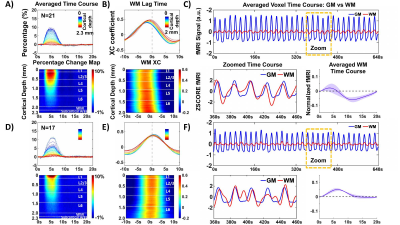 |
Distinguish hemodynamic responses at the white matter tract from the laminar-specific gray matter fMRI signal with line-scanning fMRI
Sangcheon Choi1,2, Hang Zeng1,2, Bharat Biswal3, Bruce R. Rosen4, and Xin Yu1,4
1Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Graduate Training Centre of Neuroscience, Tuebingen, Germany, 3Department of Biomedical Engineering, NJIT, Newark, NJ, United States, 4MGH/MIT/HMS Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Harvard Medical School, Massachusetts General Hospital, Charlestown, MA, United States
We applied line-scanning fMRI to investigate evoked hemodynamic responses in both laminar-specific gray matter (GM) and white matter (WM) in rats. Based on the WM-specific cross-correlation lag time to the laminar-specific fMRI signal, distinct WM hemodynamic responses were characterized across animals, showing a biphasic HRF with earlier lag times and a monophasic HRF with later lag times. Also, the lag-time dependent HRFs were detected in the subcortical area under the WM. Elucidating neurovascular coupling characteristics of distinct WM hemodynamic responses may help understand the progression of WM-related diseases, e.g. multiple sclerosis (MS) or small vessel disease (SVD).
|
 |
1355.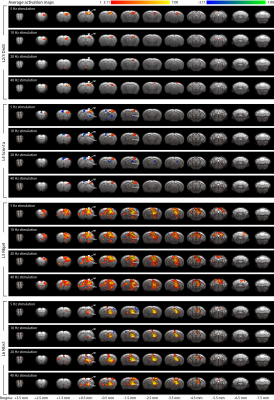 |
Layer-specific optogenetic stimulation of motor cortex activates distinct brain-wide networks
Russell W Chan1, Mazen Asaad2, Bradley J Edelman1, Hyun Joo Lee1, Hillel Adesnik3, David Feinberg3, and Jin Hyung Lee1,4,5,6
1Neurology and Neurological Sciences, Stanford University, Stanford, CA, United States, 2Molecular and Cellular Physiology, Stanford University, Stanford, CA, United States, 3Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States, 4Bioengineering, Stanford University, Stanford, CA, United States, 5Neurosurgery, Stanford University, Stanford, CA, United States, 6Electrical Engineering, Stanford University, Stanford, CA, United States
The primary motor cortex (M1) consists of a stack of interconnected but distinct layers. However, knowledge of brain-wide circuit function of M1 layer-specific pathways is lacking. Here, we combined layer-specific Cre-driver mice, optogenetics, and fMRI with subsequent electrophysiological recordings to reveal distinct M1 layer-specific networks. All L2/3, L4, L5 and L6 stimulations evoked M1 fMRI responses, while only L5 and L6 evoked robust caudate putamen and ventrolateral thalamic nucleus responses. Subsequent LFP and spike recordings were in line with these fMRI results. Overall, our techniques and results could help investigate brain-wide layer-specific cortical circuit functions in development, aging and diseases.
|
 |
1356. |
In Vivo Voltammetric Detection of Local Dopamine and Oxygen during Simultaneous BOLD fMRI
Lindsay Walton1,2,3, Matthew Verber4, Tzu-Hao Chao1,2,3, R. Mark Wightman4, and Yen-Yu Ian Shih1,2,3
1Center for Animal MRI, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Department of Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 4Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
fMRI interpretations based on traditional neurovascular coupling ignore the possible impact of vasoactive neurotransmitters released during brain activity. The striatum has atypical neurovascular coupling, and the highest concentrations of vasoactive dopamine. We evoked dopamine release in ventral striatum, and used simultaneous BOLD-fMRI and fast-scan cyclic voltammetry (FSCV) to observe global hemodynamics and quantify local dopamine and oxygen changes, respectively. Voltammetric oxygen correlated highly with BOLD, and increased linearly with local dopamine release, such that dopamine hemodynamic response functions could be derived. This multimodality explores hemodynamics at multiple spatiotemporal scales with the additional context of neurotransmission, which will improve fMRI interpretation.
|
 |
1357.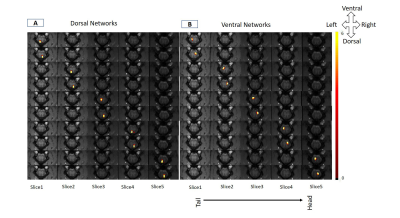 |
Intrinsic functional connectivity of spinal cord can be used to differentiate injured monkeys from normal using machine learning
Anirban Sengupta1, Arabinda Mishra1,2, Feng Wang1,2, Li Min Chen1,2,3, and John C Gore1,2,4,5,6
1Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 2Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 3Psychology, Vanderbilt University Medical Center, Nashville, TN, United States, 4Physics and Astronomy, Vanderbilt University Medical Center, Nashville, TN, United States, 5Molecular Physiology and Biophysics, Vanderbilt University Medical Center, Nashville, TN, United States, 6Biomedical Engineering, Vanderbilt University Medical Center, Nashville, TN, United States
The objective of this study was to investigate the presence of robust intrinsic networks inside the spinal cord of squirrel monkey and whether connectivity measures of these networks can detect injury in spinal cord. We used Independent Component Analysis of resting state fMRI data to obtain dorsal and ventral networks within the gray-matter of spinal cord. Within Horn Connectivity and Between Horn Connectivity measures were calculated based on the time course of Independent Components. A Support-Vector-Machine classifier could differentiate a spinal cord injured monkey from a control monkey using these connectivity measures with a low classification error of 6.67 %.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top