Scientific Session
Multimodal fMRI
Session Topic: Multimodal fMRI
Session Sub-Topic: Mechanisms of Resting-State fMRI
Oral
fMRI
| Thursday Parallel 4 Live Q&A | Thursday, 13 August 2020, 15:50 - 16:35 UTC | Moderators: Robert Barry & Patricia Figueiredo |
Session Number: O-76
1358.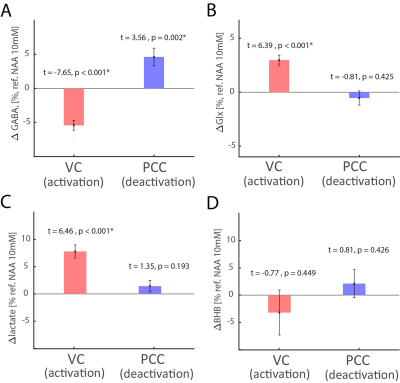 |
Metabolic basis of activated and deactivated brain network nodes in fMRI paradigms
Yury Koush1, Robin A. de Graaf1, Ron Kupers2, Laurence Dricot3, Maurice Ptito4, Kevin Behar1, Douglas L. Rothman1, and Fahmeed Hyder1
1Yale University, New Haven, CT, United States, 2University of Copenhagen, Copenhagen, Denmark, 3University of Louvain, Louvain, Belgium, 4School of Optometry, Montreal, QC, Canada
Functional MRI using blood oxygenation level dependent (BOLD) contrast identifies brain regions for task-induced (de)activation paradigms. We investigated the metabolic basis of these paradigms in activated (visual cortex, VC) and deactivated (posterior cingulate cortex, PCC) network nodes using concurrent acquisitions of J-edited lactate/GABA(γ-aminobutyric acid)/Glx(pooled glutamate and glutamine) and diffusion-weighted BOLD signal. In VC, we detected increased BOLD/lactate/glutamate, and decreased GABA, whereas in PCC BOLD decreased, GABA increased but lactate/glutamate did not change. These results suggest that BOLD responses in (de)activated areas is regulated by relatively rapid GABAergic inhibition, whereas aerobic glycolysis and glutamatergic activity dominate in activated nodes.
|
|
1359.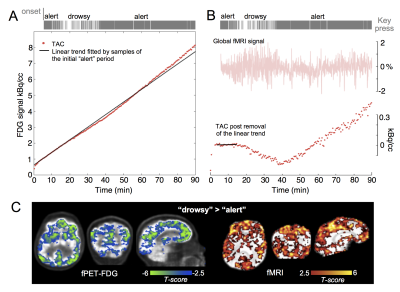 |
Employing simultaneous functional PET/MRI to map neuronal and vascular dynamics accompanying brain arousal fluctuations
Jingyuan E Chen1,2, Nina E Fultz1, Jonathan R Polimeni1,2, Ciprian Catana1,2, Bruce R Rosen1,2, Laura D Lewis3, and Christin Y Sander1,2
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States, 2Radiology, Harvard Medical School, Boston, MA, United States, 3Biomedical Engineering, Boston University, Boston, MA, United States
In this study, we investigated the feasibility of integrating simultaneous fMRI and functional PET to uncover metabolic and hemodynamic changes linked with arousal. Our findings suggested that this multi-modal toolset can reliably detect brain-wide hemodynamic and metabolic changes spanning “alert”, “drowsy” and “sleep” conditions, therefore holding great promise in disentangling arousal-induced neuronal and vascular dynamics in future investigations.
|
|
 |
1360.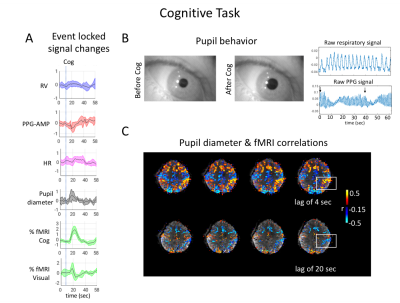 |
Variations in the sympathetic tone and fMRI signal during alert conditions
Pinar S Ozbay1, Catie Chang2, Jacco A de Zwart1, Peter van Gelderen1, and Jeff Duyn1
1NINDS, NIH, Bethesda, MD, United States, 2Vanderbilt University, Nashville, TN, United States
During light-sleep, strong correlations were observed between fMRI and peripheral signals. This can be inferred from the fingertip pulse-oximeter signal as a proxy for sympathetic activity. Sympathetic activity may also affect fMRI during wake. In this work, we analyzed data collected during cognitive tasks and deep breathing, showed strong spatio-temporal relations between pupil behavior, skin vascular tone, and fMRI signal. We demonstrate that sympathetic activity can be elicited by a variety of stimuli, that those additional measures might be useful for physiological regression and to better distinguish neuronal and autonomic contributions, which are mostly observed as anti-correlation patterns in fMRI.
|
1361.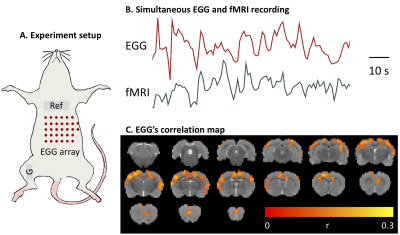 |
The Stomach and the Brain are Synchronized Intrinsically in Rats
Jiayue Cao1, Xiaokai Wang1, Kun-Han Lu2, Zhenjun Tan3, Robert Phillips3, Deborah Jaffey3, Terry Powley3, and Zhongming Liu1,2
1Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2Electrical and Computer Engineering, Purdue University, West Lafayette, IN, United States, 3Psychological Science, Purdue University, West Lafayette, IN, United States
The origins of fMRI in the resting state has been widely explored but yielded incomplete knowledge. Here, we hypothesize that gastric activity contributes to intrinsic brain activity observed with fMRI.We explored the gut-brain synchrony in rats by recording the electrogastrogram together with fMRI. We found that brainactivity is intrinsically synchronized with gastric activity at a specific resting state network in which the BOLD activity is time-locked to gastric activity with varying time delays.
|
|
 |
1362.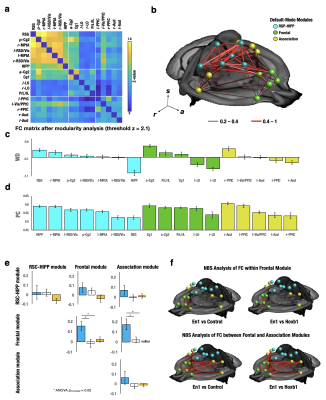 |
Locus Coeruleus derived norepinephrine alters intrinsic functional connectivity at the Default-Mode Network
Li-Ming Hsu1,2,3,4, Esteban Oyarzabal1,3,4, Manasmita Das1,3,4, Tzu-Hao Harry Chao1,3,4, Sheng Song1,3,4, Yu-Wei Chen5, Dinggang Shen2, Sungho Lee1,3,4, Patricia Jensen6, and Yen-Yu Ian Shih1,3,4
1Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Radiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Biomedical Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 4Center for Animal MRI, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 5Developmental Neurobiology, NIEHS/NIH, RDU, NC, United States, 6Developmental Neurobiology, NIH/NIEHS, RDU, NC, United States
Norepinephrine (NE) is suspected to rapidly modulate strength and structure of intrinsic functional connectivity (FC). We used chemogenetic fMRI to selectively isolate the role of NE Locus Coeruleus (LC) neurons, compared to NE A1/A2/A4 neurons, on FC modulation. Among 19 parcellated FC modules, NE-LC neurons significantly enhanced ReHo, ALFF and DC within the anterior Default-Mode, Motor and Somatosensory modules and enhanced FC strength within and between Default-Mode modules. Dynamic FC analysis found Default-Mode differences were attributed to two co-activation patterns (CAPs) associated with Default-Mode suppression that explains the ability of NE to focus wandering minds into sensory attention.
|
| 1363. | Exploring the neurovascular nature of spontaneous cerebral BOLD fluctuations in 1730 individuals – The Maastricht Study
Laura W.M. Vergoossen1,2, Jacobus F.A. Jansen1,2,3, Daan Huybrechs4, Miranda T. Schram2,5,6, Walter H. Backes1,2, and on behalf of The Maastricht Study5
1Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands, 3Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 4Computer Science, KU Leuven, Leuven, Belgium, 5Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 6School for Cardiovascular Disease, Maastricht University, Maastricht, Netherlands
In addition to spatial patterns, also temporal patterns can be identified in brain signal as non-stationary components. Fourier-transform provides only information about characteristic frequency components in dynamic brain signals and assumes that these are of stationary nature. However, brain signals are non-stationary and discrete wavelet transformation can be used to separate the signal into both frequency subbands and time-scales. In The Maastricht Study (n=1730), we found that wavelet analysis is a suitable method to demonstrate that physiological measures are associated with specific frequency subbands of the BOLD signal, and to separate the neurovascular signal into subbands representing different physiological measures.
|
|
1364.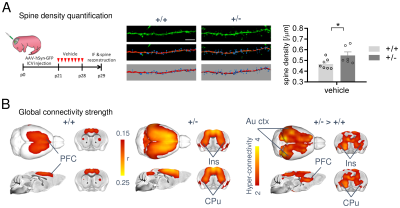 |
A cross-species link between deficient synaptic pruning and functional hyper-connectivity in autism
Marco Pagani1, Alice Bertero1,2, Alessia De Felice1, Andrea Locarno3, Ieva Miseviciute3, Stavros Trakoshis4,5, Carola Canella1,6, Elizabeth de Guzman1, Kaushtub Supekar7, Vinod Menon7, Alberto Galbusera1, Raffaella Tonini3, Michael V. Lombardo5,
Massimo Pasqualetti2, and Alessandro Gozzi1
1Functional Neuroimaging Laboratory, Istituto Italiano di Tecnologia, Rovereto, Italy, 2Biology Department, University of Pisa, Pisa, Italy, 3Neuromodulation of Cortical and Subcortical Circuits Laboratory, Istituto Italiano di Tecnologia, Genova, Italy, 4Department of Psychology, University of Cyprus, Nicosia, Cyprus, 5Laboratory for Autism and Neurodevelopmental Disorders, Istituto Italiano di Tecnologia, Rovereto, Italy, 6Center for Mind and Brain Sciences, University of Trento, Rovereto, Italy, 7Stanford University, Stanford, CA, United States
Altered brain functional connectivity is a hallmark finding in autism but the neural basis of this phenomenon remains unclear. We show that a mouse line reconstituting synaptic pruning deficits observed in postmortem autistic brains exhibits widespread functional hyper-connectivity, and that pharmacological normalization of synaptic aberrancies completely rescues behavioral and functional connectivity deficits. We also show that a similar connectivity fingerprint can be isolated in human rsfMRI scans of people with autism, and linked to overexpression of genes related to this dysfunctional pathway. Our results reveal a possible mechanistic link between deficient synaptic pruning and functional hyper-connectivity in autism.
|
|
 |
1365.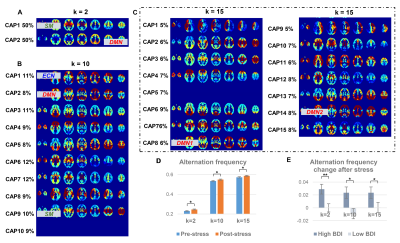 |
Transition Frequencies across the Brain States under Stress Differentiate Depression Vulnerability
Xue Zhang1,2, Hua Guo1, and Lihong Wang3
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Radiological Sciences Laboratory, Department of Radiology, Stanford University, Palo Alto, CA, United States, 3Department of Psychiatry, University of Connecticut School of Medicine, Farmington, CT, United States
Brain state transitions during resting-state reflect the variation of the baseline homeostasis, it is still unclear how the state interactions are modulated under stress. In the current study, the stress-induced change of the co-activation pattern transitions was examined in two independent cohorts by scanning resting-state fMRI pre- and post- a math task, its association with depression vulnerability was also explored. The post- versus pre-stress resting-state comparison showed an increased state transition frequency under stress, and those with higher depression scores shifted more post-stress in both cohorts, indicating the disturbed brain homeostasis under stress and lower recovery ability from stress.
|
1366.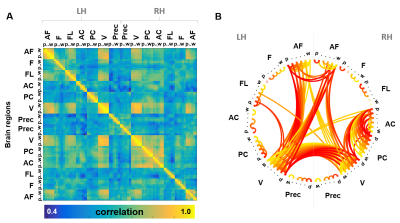 |
Cross-cortical Depth-dependent Interactions in the Human Brain using EPIK
Patricia Pais-Roldán1, Seong Dae Yun1, Michael Schwerter1, and Jon N Shah1,2,3,4
1Forschungszentrum Jülich - INM-4, Jülich, Germany, 2Forschungszentrum Jülich - INM-11, Jülich, Germany, 3JARA-BRAIN, Aachen, Germany, 4RWTH Aachen University, Aachen, Germany
Cross-cortical interactions in the human brain remain poorly understood and are often over-simplified as a 2-dimensional cortical model in fMRI studies. To date, high-resolution fMRI has been limited to relatively small brain slabs that cover particular areas of interest, providing fine mapping of local circuits but precluding macroscale analysis. Here, an EPIK sequence was used to measure the GE-BOLD signal from individual cortical layers through most of the brain. The combination of high resolution (0.63 mm isotropic) and large coverage fMRI enabled identification of long-distance neuronal interactions that take place between particular cortical depths during resting-state.
|
|
 |
1367.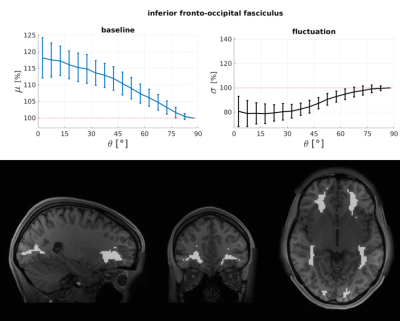 |
White matter resting-state BOLD signals depend on the orientation of the local diffusion tensor axis relative to the B0-field
Olivia Viessmann1, Qiyuan Tian1, Michaël Bernier1, David H Salat1,2, and Jonathan Rizzo Polimeni1,3
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2VA Boston Healthcare System, Boston, MA, United States, 3Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
We aimed to test whether the amplitudes of resting-state BOLD signals within the white matter depend on the orientation of the local diffusion tensor relative to the B0-field. This was assessed using resting-state BOLD and diffusion data provided by the HCP. Baseline BOLD signals were about 11% higher in voxels where primary DTI directions were parallel to B0 compared to perpendicular. Because myelinated fibres will change local tissue T2*, which will also impact the BOLD signal, we tested whether the observed BOLD orientation effect was driven by static effects on the baseline or dynamic effects from changes in blood oxygenation.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top