Oral - Power Pitch Session
Novel Imaging Techniques for CMR
Session Topic: Novel imaging techniques for CMR
Session Sub-Topic: Cardiovascular Power Pitch: Technical
Oral - Power Pitch
Cardiovascular
| Thursday Parallel 3 Live Q&A | Thursday, 13 August 2020, 14:20 - 15:05 UTC | Moderators: Yang Yang |
Session Number: PP-09
 |
1087.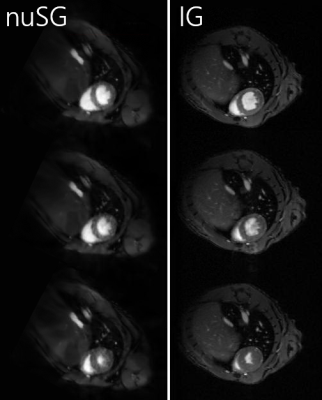 |
Retrospective Compensation of Cardiac and Respiratory Motion in Mice using nonuniform Self-Gating
Tobias Speidel1, Patrick Metze2, Fabian Straubmueller2, Hao Li1, and Volker Rasche2
1Core-Facility Small Animal Imaging, Ulm University, Ulm, Germany, 2Experimental Cardiovascular MRI, Ulm University Medical Center, Ulm, Germany
The application of self-gating techniques to small animal imaging poses challenging problems, particularly dominated by the high respiratory frequencies. Established self-gating methods are based on information that is extracted either from the k-space itself or from low-resolution images, leading to one-dimensional gating signals. These approaches are prone to fail in the case of arrhythmic respiratory and/or cardiac motion. The concept of nonuniform self-gating is capable of retrospectively considering respiratory and cardiac motion despite significant changes in cardiac or respiratory frequencies by using a two-dimensional gating matrix for deriving the required gating information.
|
 |
1088.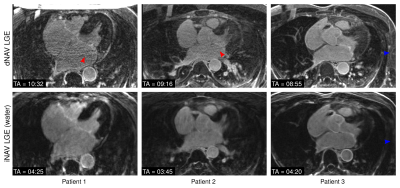 |
Highly efficient respiratory motion-compensated 3D water/fat late gadolinium enhanced atrial wall imaging
Camila Munoz1, Iain Sim2, Aurelien Bustin1, Radhouene Neji1,3, Karl P Kunze3, Michaela Schmidt4, Mark O’Neill2, Steven E Williams2, Rene M Botnar1, and Claudia Prieto1
1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Cardiovascular Imaging Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4Cardiovascular MR Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
3D late gadolinium enhanced (LGE) imaging is a promising technique for the non-invasive assessment of atrial fibrosis. In order to minimize respiratory motion, current 3D LGE atrial imaging relies on diaphragmatic navigator gating, leading to time-consuming scans with unpredictable duration. Here we introduce a highly efficient respiratory motion-compensated 3D water/fat IR-prepared LGE atrial imaging protocol with predictable scan time. Preliminary results demonstrate that the proposed approach enables depiction of atrial scar comparable to conventional 3D atrial LGE imaging, but with a significantly shorter scan time of <5 minutes. The proposed approach holds promise for high-resolution atrial wall imaging.
|
 |
1089.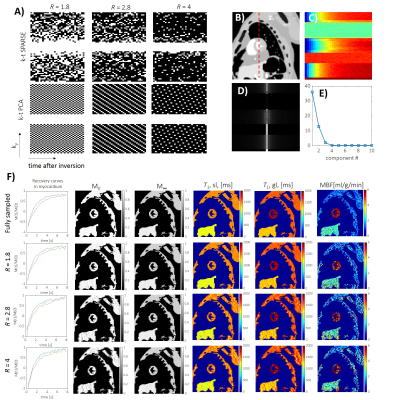 |
Accelerating Myocardial Arterial Spin Labeling in Small Animals by Exploiting Spatiotemporal Correlations
Grzegorz Kwiatkowski1, Frank Kober2, and Sebastian Kozerke1
1Institute for Biomedical Engineering, ETH Zurich, Zurich, Switzerland, 2Aix Marseille Univ, CNRS, CRMBM, Marseille, France
The feasibility of accelerating arterial spin labelling (ASL) by exploiting spatiotemporal correlations for assessing myocardial perfusion in small animals is demonstrated. Based on numerical simulations and retrospectively undersampled in-vivo data, three-fold acceleration yields errors below 16 ± 10 % in myocardial blood flow quantification and hence the method is considered promising to shorten the long scan times of myocardial ASL in small animals.
|
1090.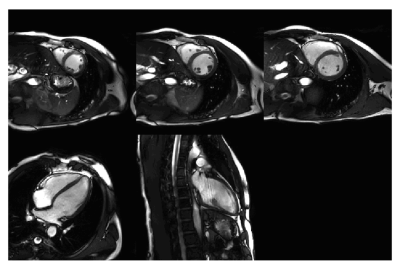 |
Real-Time free breathing cardiac CINE MRI with 84 channel high density receive array at 3 Tesla: Initial experience
Mark Gosselink1, Hugo Klarenberg2, Hildo J. Lamb3, Gustav J. Strijkers2, Dennis W.J. Klomp1, Tim Leiner1, and Martijn Froeling1
Video Permission Withheld
1University Medical Center Utrecht, Utrecht, Netherlands, 2Amsterdam University Medical Center, Amsterdam, Netherlands, 3Leiden University Medical Center, Leiden, Netherlands
Cardiac triggered CINE imaging is used clinically for the assessment of cardiac function. The purpose of this study is to investigate the feasibility of real time free breathing CINE MRI using a high density receive array on a 3T clinical system with online compressed SENSE image reconstruction. We demonstrate feasibility of real-time 2D CINE imaging using a high-density coil array.
|
|
1091.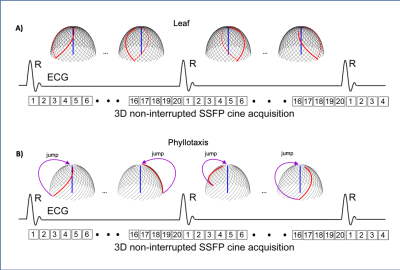 |
Leaf: A Novel 3D Radial Trajectory for Free-breathing 3D Cine Cardiac Magnetic Resonance Imaging
Lukas Braunstorfer1, Mehdi H. Moghari2, and Andrew H. Powell3
1Cardiology, Harvard Medical School, cambridge, MA, United States, 2Cardiology, Harvard Medical School, boston, MA, United States, 3Cardiology, Harvard Medical School, Boston, MA, United States
Free-breathing 3D cine steady-state free precession (SSFP) sequence with radial phyllotaxis trajectory is recently performed for making cardiac magnetic resonance imaging (MRI) exams easy and more comfortable for patients. Phyllotaxis trajectory is susceptible to the eddy current artifact due to a large gradient change during the 3D cine SSFP acquisition for measuring the centerline of k-space. We, therefore, developed and validated a novel leaf trajectory that minimizes the gradient change, and eddy current.
|
|
1092.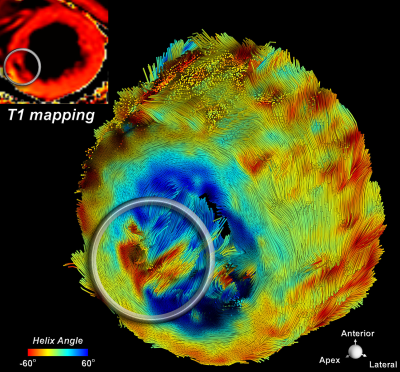 |
Cardiac Diffusion Tensor MRI Using M2-gSlider with a Real-Time Slice Tracking Respiratory Navigator
Christopher Nguyen1,2,3, Timothy G Reese3,4, Congyu Liao3,4, William J Kostis5, Marcel P Jackowski6, Kawin Setsompop3,4, and Choukri Mekkaoui3,4
1Cardiovascular Research Center, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Medicine, Harvard Medical School, Boston, MA, United States, 3Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Cardiovascular Institute, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, United States, 6Department of Computer Science, University of São Paulo, São Paulo, Brazil
Free-breathing isotropic cardiac diffusion tensor MRI (DT-MRI) of the left ventricle can be performed using second moment (M2) motion compensated spin echo encoding and generalized slice dithered enhanced resolution (gSlider). This technique provides substantial improvements in spatial resolution and consequently in the accuracy of diffusion-based indices. However, M2-gSlider’s RF slice encoding is susceptible to through-slice motion, limiting the maximal improvement in slice resolution. Here, we evaluate the addition of a slice tracking respiratory navigator (NAV) to prospectively adjust slice position in real-time. M2-gSlider-NAV was validated in healthy volunteers and tested in a patient with a history of myocardial infarction.
|
|
1093.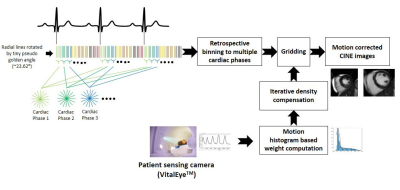 |
Rapid free breathing multi-slice radial CINE MRI using a patient sensing camera
Guruprasad Krishnamoorthy1,2, Joao Silva Tourais1,2, Jouke Smink1, Marc Kouwenhoven1, and Marcel Breeuwer1,2
1Philips Healthcare, Best, Netherlands, 2Eindhoven University of Technology, Eindhoven, Netherlands
The benefits of the current cardiac CINE MRI are often limited by the requirement of patient co-operation for multiple breath-holds. To overcome this limitation, we present a new, free-breathing respiratory motion-compensated 2D multi-slice radial CINE method for left ventricular functional assessment. Our method utilizes the respiratory signal obtained from a patient sensing camera for performing motion weighted density compensation in radial gridding to minimize respiratory motion artifacts in the reconstructed image. The left-ventricular functional assessments from volunteers obtained using the proposed method are in good agreement with the results obtained using the standard Cartesian breath-hold method.
|
|
 |
1094.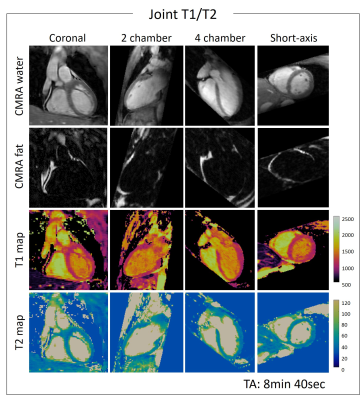 |
3D Whole-heart High-resolution Motion Compensated Joint T1/T2 Mapping
Giorgia Milotta1, Aurelien Bustin1, Olivier Jaubert1, Radhouene Neji1, Claudia Prieto1, and Rene Botnar1
1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Tissue characterization including identification and quantification of fibrosis and oedema plays an important role in many myocardial diseases. Conventionally 2D T1 and T2 maps are acquired sequentially under several breath-holds. However these approaches achieve limited spatial resolution and coverage. Furthermore, partial volume effects at water-fat interfaces may affect T1 and T2 quantification. In this work, we propose a free-breathing high-resolution whole-heart joint T1 and T2 mapping sequence with Dixon encoding which provides co-registered 3D T1 and T2 maps and complementary 3D anatomical water coronary magnetic resonance angiography (CMRA) and fat images in a single scan of ~9min.
|
 |
1095.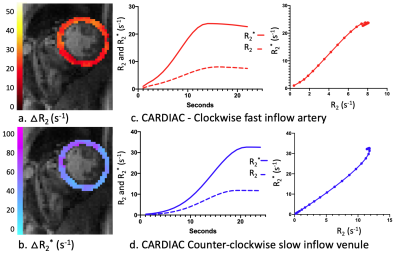 |
Vessel architectural imaging in the human heart using heartbeat-to-heartbeat GESE-EPI
Maaike van den Boomen1,2, Mary Kate Manhard1,3, Kyrre E. Emblem4, David E. Sosnovik1,5,6, Niek H.J. Prakken2, Christopher Nguyen1,5,6, Kawin Setsompop1,3,7, and Ronald J.H. Borra2,8
1A.A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States, 2Department of Radiology, University Medical Center Groningen, Groningen, Netherlands, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Department of Diagnostic Physics, Oslo University Hospital, Oslo, Norway, 5Cardiovascular Research Center, Massachusetts General Hospital, Boston, MA, United States, 6Department of Medicine, Harvard Medical School, Boston, MA, United States, 7Division of Health Sciences and Technology, Harvard-MIT, Cambridge, MA, United States, 8Department of Nuclear Medicine and Molecular Imaging, University Medical Center Groningen, Groningen, Netherlands
Vessel architectural imaging (VAI) is explored in the heart by using a heartbeat-to-heartbeat GESE-EPI sequence upon injection of Gd-DTPA. Cardiac VAI can provide the vascular type, caliber, density and blood volume fraction indices in the myocardium, in line with previous work performed in the brain. Further histological validation of these indices is needed, but our initial results demonstrates the feasibility of this technique to advance cardiovascular research into cardiac microvascular dysfunction.
|
 |
1096.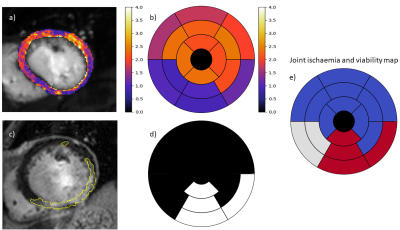 |
Fully automated assessment of myocardial ischemic burden – a joint perfusion and viability mapping approach
Cian Michael Scannell1, Adriana Villa1, Stefano Figliozzi1, Jack Lee1, Mikto Veta2, Marcel Breeuwer2,3, and Amedeo Chiribiri1
1King's College London, London, United Kingdom, 2Eindhoven University of Technology, Eindhoven, Netherlands, 3Philips Healthcare, Best, Netherlands
Quantitative myocardial perfusion MRI has the potential to guide the management of patients with coronary artery disease. It has been shown to have high prognostic value and has the benefit of being automated and user-independent. However, a known limitation of the technique is that it cannot distinguish between perfusion defects that are due to a previous infarction and inducible ischemia. In this work we combine quantitative myocardial perfusion with a further automated pipeline for scar quantification from LGE images. It is shown that this combined assessment can identify areas of inducible ischemia in which the tissue is viable.
|
 |
1097.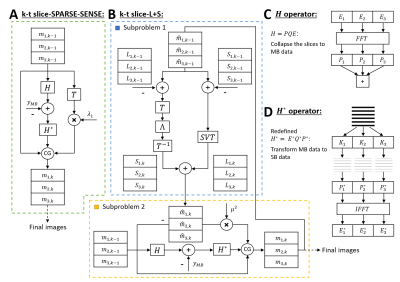 |
Multiband first-pass myocardial perfusion MRI using a slice-low-rank plus sparse model
Changyu Sun1, Austin Robinson2, Christopher Schumann2, Daniel Weller1,3, Michael Salerno1,2,4, and Frederick Epstein1,4
1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Medicine, University of Virginia, Charlottesville, VA, United States, 3Electrical and Computer Engineering, University of Virginia, Charlottesville, VA, United States, 4Radiology, University of Virginia, Charlottesville, VA, United States
Multiband (MB) excitation and in-plane acceleration of first-pass perfusion imaging has the potential to provide a high aggregate acceleration rate. Our recent slice-SPIRiT work formulated MB reconstruction as a constrained optimization problem that jointly uses in-plane and through-plane coil information and MB data consistency. Here we extend these methods to develop k-t slice-SPARSE-SENSE and k-t slice-L+S reconstruction models. First-pass perfusion data with MB=3 and rate-2 k-t Poisson-disk undersampling were acquired in 6 patients. The slice-L+S reconstruction showed sharper borders and greater contrast than slice-SPARSE-SENSE and had better image quality scores as assessed by two cardiologists.
|
1098.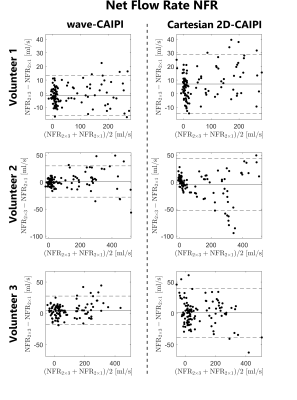 |
Accelerated 4D Flow MRI with wave-CAIPI
Julian A. J. Richter1,2, Tobias Wech1, Andreas M. Weng1, Manuel Stich1,3, Ning Jin4, Thorsten A. Bley1, and Herbert Köstler1
1Department of Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany, 2Comprehensive Heart Failure Center Würzburg, Würzburg, Germany, 3Siemens Healthcare, Erlangen, Germany, 4Siemens Medical Solutions USA, Inc., Chicago, IL, United States
The wave-CAIPI technique was applied to aortic 4D flow MRI. Three healthy volunteers were examined and flow parameters as well as hemodynamic flow patterns were derived from the measured data. The acquisitions were retrospectively accelerated and compared to conventional Cartesian 2D-CAIPI sampling. Using wave-CAIPI sampling, the deviations between flow parameters of the 6-fold accelerated scans and the references (2-fold accelerated) could be reduced by up to 47% compared to Cartesian sampling. As a consequence, the acquisition time of aortic 4D flow acquisitions could be decreased to 3.5 minutes with higher precision, concerning the calculated flow parameters and hemodynamic flow patterns.
|
|
1099.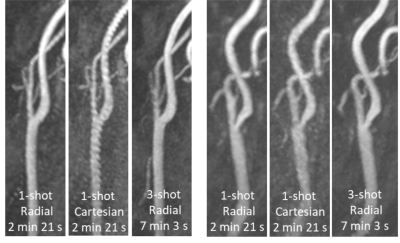 |
Feasibility of Rapid Quiescent-Interval Slice-Selective MRA of the Carotid Arteries Using Radial Sampling and Deep Learning Reconstruction
Ioannis Koktzoglou1,2, Rong Huang1, Pascale J Aouad1,3, Emily A Aherne1,3, Archie L Ong2,4, and Robert R Edelman1,3
1Radiology, NorthShore University HealthSystem, Evanston, IL, United States, 2The University of Chicago Pritzker School of Medicine, Chicago, IL, United States, 3Radiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 4Neurology, NorthShore University HealthSystem, Evanston, IL, United States
Ungated quiescent-interval slice-selective (QISS)-based magnetic resonance angiography (MRA) of the extracranial carotid arteries normally carries scan times of approximately 7 minutes. This work evaluated the feasibility of 3-fold accelerated single-shot QISS MRA in under three minutes using radial k-space sampling and a patch-based deep learning image reconstructive strategy.
|
|
1100.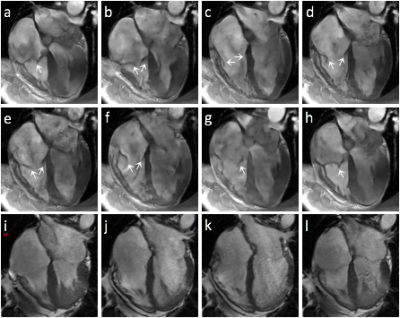 |
On the Feasibility of Noncontrast Valvular Cine MRI with High Spatial Resolution and High Frame Rate Using Deep-learning-powered Acceleration
Peng Lai1, Christopher M Sandino2, Shreyas S Vasanawala3, Anne Menini1, Haonan Wang4, Anja C.S Brau1, and Martin A Janich5
1GE Healthcare, Menlo Park, CA, United States, 2Electrical Engineering, Stanford University, Palo Alto, CA, United States, 3Radiology, Stanford University, Palo Alto, CA, United States, 4GE Healthcare, Waukesha, WI, United States, 5GE Healthcare, Munich, Germany
Valvular imaging is challenging to conventional cine MRI due to its requirement of very high spatial and temporal resolution. This work preliminarily investigated valvular cine MRI with highly accelerated data acquisition powered by deep learning reconstruction. Our results demonstrated the feasibility to resolve valve anatomy and motion with nearly 1mm spatial resolution and 10ms frame rate, while flow-induced dephasing generates shading in blood pool and can complicate valve visualization.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top