Digital Posters
Imaging Metabolites: CEST & MT
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 6 | 13:00 - 14:00 |
 |
3693.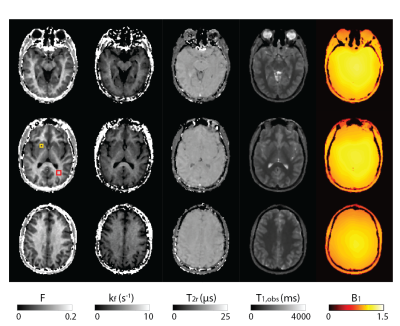 |
Whole-brain B1-corrected quantitative MT imaging in less than 5 minutes
Roya Afshari1,2, Francesco Santini1,2, Rahel Heule3,4, Craig Meyer5, Josef Pfeuffer6, and Oliver Bieri1,2
1Department of Radiology, Division of Radiological Physics, University Hospital Basel, Basel, Switzerland, 2Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 3Department of High Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 4Department of Biomedical Magnetic Resonance, University of Tübingen, Tübingen, Germany, 5Department of Biomedical Engineering, University of Virginia, Virginia, VA, United States, 6Department of Application Development, Siemens Healthcare, Erlangen, Germany
To develop a rapid quantitative magnetization transfer (qMT) imaging methodology that offers whole brain coverage and takes into account B1-field inhomogeneities for improved parameter estimation accuracy in the clinical setting.
|
||
3694.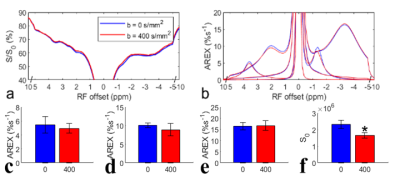 |
Insignificant contribution of blood to NOE(-1.6)
Jing Cui1, Yu Zhao1, Feng Wang1, Junzhong Xu1, Daniel Gochberg1, John Gore1, and Zhongliang Zu1
1Vanderbilt University Medical Center, Nashville, TN, United States
We evaluated the contribution of blood to the NOE(-1.6) from rat brains by using two blood suppression approaches: (1) signal acquisition with a diffusion-weighting of b=400s/mm2; (2) intravascular injection of 5mg/kg MION, and by measurements on an ex vivo blood sample. Results show that the NOE(-1.6) does not change significantly with diffusion weighting and is relatively weaker in ex vivo blood than that in brain, but decreases significantly in vivo after injection of MION. This study suggests that NOE(-1.6) is not mainly from blood, and that MION particles alter the NOE(-1.6) but have much weaker effects on other CEST effects.
|
|||
3695.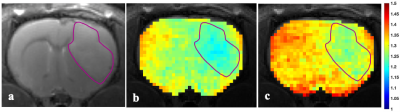 |
The Effect of Cariporide on Tumour Intracellular pH: A Study in Rat C6 Glioma using AACID-CEST-MRI
Maryam Mozaffari1,2, Nivin Nystrom1,2, Alex Li1, Miranda Bellyou1, Timothy Scholl1,2, and Robert Bartha1,2
1Robarts Research Institute, London, ON, Canada, 2Department of Medical Biophysics, Western University, London, ON, Canada
The results of this study suggest the non-invasive assessment of tissue pH may provide value for monitoring the progression of diseases such as brain cancer that involve pH modification. This study also demonstrates that tissue acidification in a rat C6 glioma model and in contralateral tissue can be measured following drug injection by endogenous pH-weighted contrast produced by CEST-MRI. It is noteworthy that drugs like cariporide can acidify tumours and normal tissue. However, such drugs could enhance the efficacy of existing standard treatments in different human malignancies.
|
|||
3696.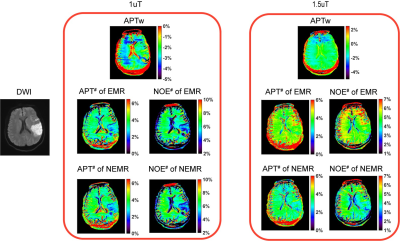 |
Numerical Fit of Extrapolated Semisolid Magnetization Transfer Reference Signal (NEMR) for Improved pH-Weighted Imaging of Ischemic Stroke
Xingwang Yong1, Shanshan Lu2, Yi-Cheng Hsu3, Yi Sun3, Dan Wu1, and Yi Zhang1
1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, Zhejiang, China, 2The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China, 3MR Collaboration, Siemens Healthcare Ltd., Shanghai, China
Amide proton transfer (APT) imaging can detect pH-related changes in ischemic stroke lesions. However, the widely-used magnetization transfer ratio asymmetry (MTRasym) analysis method is susceptible to various contamination sources. Here we improved the previous extrapolated semisolid magnetization transfer reference (EMR) method by numerical fitting of the EMR signal (NEMR) in the modified Bloch-McConnel equation. The proposed NEMR method was compared with the previous EMR method in Monte Carlo simulations, demonstrating superior accuracy. Furthermore, the NEMR maps were compared with EMR and MTRasym maps in nine ischemic stroke patients, yielding a better depiction of the ischemic lesions.
|
|||
3697. |
Promising nerve imaging biomarkers for applications in inherited neuropathies
Alison R Roth1, Jun Li2, and Richard Dortch1
1Neuroimaging Research, Barrow Neurological Institute, Phoenix, AZ, United States, 2Neurology, Wayne State University, Detroit, MI, United States
Five magnetization transfer (MT) imaging metrics extracted from the sciatic nerve (magnetization transfer ratio (MTR), cross-sectional area (CSA), circularity, eccentricity, and nerve fascicle density) were assessed for their ability to act as imaging biomarkers in patients with inherited neuropathies.
|
|||
3698.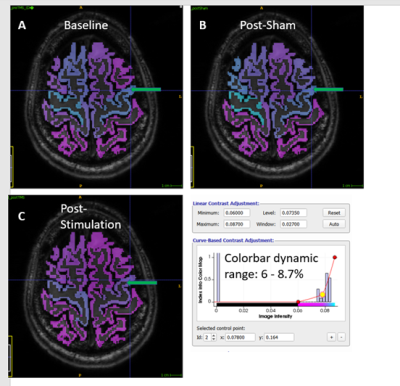 |
Using Glutamate-Weighted MR Imaging (GluCEST) to Detect Effects of Transcranial Magnetic Stimulation
Abigail T.J. Cember1, Benjamin Deck2, Jared Zimmerman3, Brian Erickson2, Apoorva Kelkar2, Olufunsho Faseyitan3, Mark Elliott1, Ravinder Reddy1, and John D. Medaglia2
1Center for Magnetic Resonance and Optical Imaging, Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Psychology, Drexel University, Philadelphia, PA, United States, 3Laboratory for Cognition and Neural Stimulation, Department of Neurology, University of Pennsylvania, Philadelphia, PA, United States
GluCEST was used to measure the effects of transcranial magnetic stimulation (TMS) to the motor cortex in healthy volunteers. Volunteers were scanned before and after stimulation or a sham, non-stimulating procedure. The data were analyzed by registering gluCEST maps to a cortical gray matter segmentation from Freesurfer and performing regional analysis. We find that this form of TMS, known as continuous theta-burst stimulation, had a consistent but non-local effect of decreasing the gluCEST signal in the brains of volunteers who received the stimulation in comparison to those who received a sham (placebo) procedure.
|
|||
3699.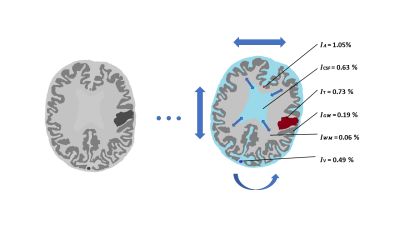 |
A digital human head phantom for validation of retrospective motion correction in glucoCEST MRI
Patrick M. Lehmann1, Mads Andersen2, Anina Seidemo1, Xiang Xu3,4, Xu Li4,5, Nirbhay Yadav4,5, Ronnie Wirestam1, Frederik Testud6, Patrick A. Liebig7, Pia C. Sundgren8,9, Peter C. M. van Zijl4,5, and Linda Knutsson1,4
1Department of Medical Radiation Physics, Lund University, Lund, Sweden, 2Philips Healthcare, Copenhagen, Denmark, 3BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 6Siemens Healthcare AB, Malmö, Sweden, 7Siemens Healthcare GmbH, Erlangen, Germany, 8Department of Radiology, Lund University, Lund, Sweden, 9Lund University Bioimaging Centre, Lund University, Lund, Sweden
D-glucose was recently suggested as a CEST-based biodegradable alternative to gadolinium-based contrast agents. Dynamic glucose-enhanced (DGE) MRI can retrieve information about glucose uptake, determined by tissue perfusion, transport, and metabolism. Motion artefacts in DGE-MRI can be mistaken for CEST effects, while motion correction may erroneously alter true DGE signal. A digital human head phantom based on a realistic glucose infusion protocol was developed to analyse motion artefacts and validate rigid-head retrospective motion correction. This phantom can be used for testing different correction approaches using various motion patterns and contrast responses to better understand these effects in vivo.
|
|||
3700. |
Volumetric glutamate-weighted MR imaging (gluCEST) enables in vivo detection of metabolic differences between human hippocampal subfields
Abigail T.J. Cember1, Ravi Prakash Reddy Nanga1, Hari Hariharan1, Neil E. Wilson2, Puneet Bagga3, and Ravinder Reddy1
1Center for Magnetic Resonance and Optical Imaging, Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Siemens Medical Solutions, USA, Malvern, PA, United States, 3Department of Diagnostic Imaging, St. Jude Children's Research Hospital, Memphis, TN, United States
We performed volumetric (3D) gluCEST with novel post-processing to image a slab containing the medial temporal lobe (MTL) in healthy human subjects. This data was segmented using a 7T atlas for hippocampal subfields. Upon further regional analysis, it was found that statistically significant differences exist between the mean gluCEST of MTL subfields; most prominently, that the dentate gyrus has greater gluCEST contrast than neighboring regions. We believe after some preliminary validation that this trend is independent of the B1 distribution or other variables. Moreover, it corroborates existing models of brain physiology identifying the DG as the locus of neurogenesis.
|
|||
3701.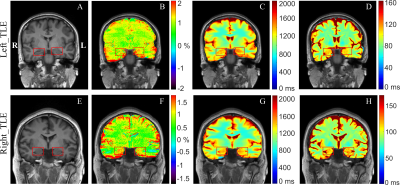 |
Automated CEST Measurements for the Lateralization of Epileptic Foci in Temporal Lobe Epilepsy at 3 T
Qingqing Wen1, Kang Wang2, Wenqi Wang1, Yi Sun3, Dan Wu1,2, and Yi Zhang1,2
1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 2Department of Neurology, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China, 3MR Collaboration, Siemens Healthcare Ltd., Shanghai, China
Chemical Exchange Saturation Transfer (CEST) imaging at 3T was applied to patients with temporal lobe epilepsy (TLE). Hippocampus and amygdala were automatically segmented, and then the magnetization transfer ratio asymmetry (MTRasym), T1, and T2 values in these regions of interest were determined. It was found that MTRasym in the hippocampus and amygdala was much more accurate than the quantitative T1 and T2 maps in predicting the seizure laterality, with an AUC value of 0.80 and a success rate of 17/20. The study indicated that CEST at 3T could potentially aid the clinical assessment of the epileptic foci in TLE patients.
|
|||
3702.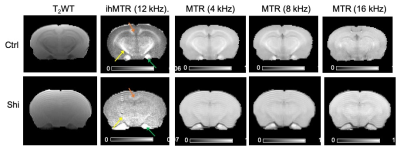 |
ihMT Analysis of Myelin in the Shiverer Mouse Brain
Choong Heon Lee1, Piotr Walczak2, and Jiangyang Zhang1
1Radiology, NYU Medical Center, New York, NY, United States, 2University of Maryland School of Medicine, Baltimore, MD, United States
Although multiple MRI techniques have been developed to detect and monitor myelin loss and repair, their sensitivity to myelin is often impaired by the underlying pathology. In this study, we demonstrate that inhomogeneous magnetization transfer (ihMT) can detect residual non-compact myelin in the context of hypercellular white matter in the dysmyelinating shiverer mouse model with optimized imaging parameters, whereas conventional MT show no apparent contrast. This result suggests that ihMT has higher sensitivity to myelin than conventional MT in the presence of cell infiltrations.
|
|||
3703.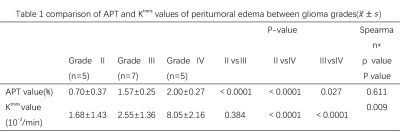 |
The value of Amide Proton Transfer weighted and Dynamic Contrast-Enhanced imaging in peritumoral edema assisted grading of gliomas
Xinying Ren1, Yujing Li1, Rui Wang1, Tao Wen1, Diaohan Xiong1, Pengfei Wang1, Guangyao Liu1, Jing Zhang1, and Kai Ai2
1Department of Magnetic Resonance, Lanzhou University Second Hospital, Lanzhou, China, 2Philips Healthcare, Xi'an, China
The study was to quantitatively analyze the peritumoral infiltration assisted gliomas grading by using amide proton transfer (APT) and dynamic contrast-enhanced imaging (DCE). Although many scholars use APT and DCE to help grading gliomas, but few of them pay attention to the peritumoral area. This research investigated the relationship between APT and DCE based Ktrans value in peritumoral. The results show that these two parameters are significantly correlated. Therefore, the APT and Ktrans value of peritumor edema in gliomas may be used to differentiate glioma grades, and APT value performs better in distinguishing grade Ⅱ and Ⅲ.
|
|||
3704.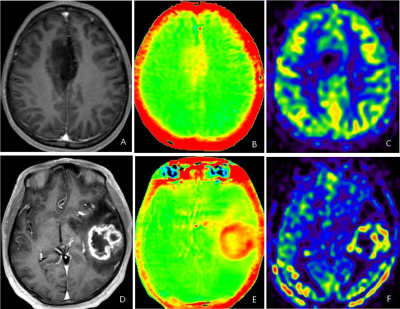 |
Discrimination of IDH1 Genotype and 1p/19q Status in Glioma: A comparison study between Arterial Spin Labeling and Amide Proton Transfer imaging
DiaoHan Xiong1, Rui Wang1, Tao Wen1, Yujing Li1, Xinying Ren1, Pengfei Wang1, Guangyao Liu1, Jing Zhang1, and Kai Ai2
1Department of Magnetic Resonance, Lanzhou University Second Hospital, Lanzhou, China, 2Philips Healthcare, Xi'an, China
We investigated the association of amide proton transfer weighted imaging (APTw) and 3D arterial spin label (3D-pCASL) with IDH1 Genotype and 1p/19q Status in gliomas,then compared the two methods. All patients underwent MR examination, including APT and 3D-pCASL scan to get their values of the solid part. The results showed both APTw and 3D-pCASL had predictive values on IDH1 Genotype and 1p/19q Status in gliomas, and the performance of 3D-pCASL was better. We conclude that both APTw and 3D-pCASL can be used to predict the gene type of IDHin high-grade and 1p/19q co-deletion of gliomas before surgery, meanwhile 3D-pCASL is the better choice.
|
|||
3705.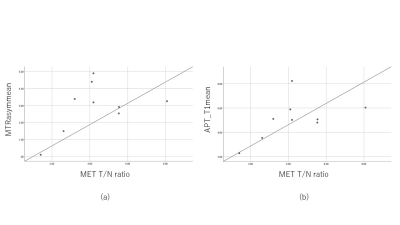 |
Potential feasibility of new parameters on CEST imaging by multi pool model in relation to 11C-MET uptake on PET/CT and IDH1 mutation in gliomas
Yasukage Takami1, Naruhide Kimura1, Katsuya Mitamura1, Takashi Norikane1, Keisuke Miyake2, Tatsuya Yamasaki3, Kazuo Ogawa3, Mitsuharu Miyoshi4, and Yoshihiro Nishiyama1
1Department of Radiology, Faculty of Medicine, Kagawa University, Miki-cho, Japan, 2Department of Neurological Surgery, Faculty of Medicine, Kagawa University, Miki-cho, Japan, 3Department of Clinical Radiology, Kagawa University Hospital, Miki-cho, Japan, 4Global MR Applications & Workflow, GE Healthcare Japan, Hino-shi, Japan
The purpose of this study was to evaluate the correlation between parameters on CEST imaging by multi pool model and 11C-methionine (MET) uptake on PET/CT in gliomas. The maximum and mean values of the parameters on CEST imaging were measured. 11C-MET uptake was semiquantitatively assessed using tumor-to-contralateral normal brain tissue (T/N) ratio. Several correlation coefficients between MTRasym and T/N ratio, and APT_T1 and MET T/N ratio were relatively high, but not statistically significant. These preliminary results suggest that parameters on CEST imaging by multi pool model seems to correlate with 11C-MET uptake on PET/CT in patients with gliomas.
|
|||
3706.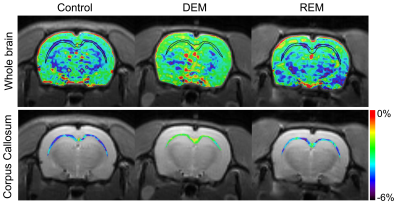 |
Amide proton transfer-weighted MR imaging in the rat brain of demyelination and remyelination
Do-Wan Lee1, Hwon Heo2, Chul‐Woong Woo3, Jae-Im Kwon3, Joongkee Min3, Monica Young Choi2, Yeon Ji Chae2, Dong‐Cheol Woo2,3, Kyung Won Kim1, Jeong Kon Kim1, Hyo Jeong Chin4, and Dong‐Hoon Lee4
1Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea, Republic of, 2Department of Convergence Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea, Republic of, 3Convergence Medicine Research Center, Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea, Republic of, 4Department of Radiological Science, College of Health Sciences, Yonsei University, Wonju, Korea, Republic of
Investigations of amide proton signal changes in the white matter of demyelinating diseases may provide important biophysical information for diagnostic and prognostic assessments. We attempted to evaluate in vivo APTw signal changes within the CC in a reversible cuprizone-induced demyelination rat model using amide proton transfer-weighted (APTw) MRI at 7-T. We also used immunohistochemical staining to characterize demyelinating and remyelinating activity in the CC following myelin and axon changes. Significant APTw metric changes coupled with the histological characteristics of the demyelination and remyelination processes indicate the potential usefulness of APTw 7T MRI to monitor earlier myelination processes.
|
|||
3707.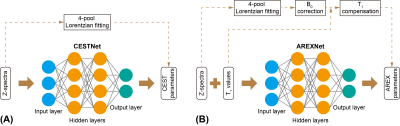 |
CEST and AREX data processing based on deep neural network: application to image Alzheimer’s disease at 3T
Jianpan Huang1, Joseph H. C. Lai1, Kai-Hei Tse2, Gerald W.Y. Cheng2, Xiongqi Han1, Yang Liu1, Zilin Chen1, Lin Chen3,4, Jiadi Xu3,4, and Kannie W. Y. Chan1,4,5
1Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, China, 2Department of Health Technology and Informatics, The Hong Kong Polytechnic University, Hong Kong, China, 3F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Research Institute, Baltimore, MD, United States, 4Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5City University of Hong Kong Shenzhen Research Institute, Shenzhen, China
Chemical exchange saturation transfer (CEST) magnetic resonance imaging (MRI) is a promising molecular imaging technology. Apparent exchange-dependent relaxation (AREX) provides CEST contrast with less influence of T1. Here, deep neural network based CEST/AREX analysis methods (CESTNet/AREXNet) were applied to analyze the CEST data of normal and AD mouse brains at 3T. Significant lower amide proton transfer/magnetization transfer (APT/MT) signals related to amyloid β-peptide (Aβ) plaque depositions, which were validated by immunohistochemistry results, were detected in Alzheimer’s disease (AD) mouse brains compared to age-matched wild type (WT) mouse brains. The well-established CESTNet/AREXNet have great potential to facilitate AD identification at 3T.
|
|||
3708.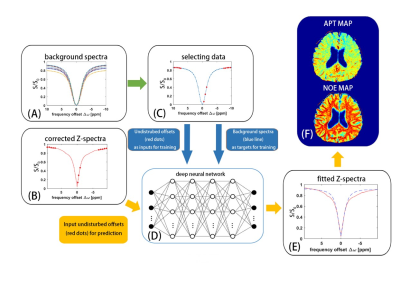 |
CEST imaging with neural network fitting of the human brain at 3T
Zhichao Wang1, Yu Zhao2, Xu Yan3, Zhongshuai Zhang3, Caixia Fu3, Hui Tang4, and Jianqi Li1
1Shanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronic Science, East China Normal University, Shanghai, China, 2Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 3MR Collaboration NE Asia, Siemens Healthcare, Shanghai, China, 4Department of Radiology, Renji Hospital affiliated to Shanghai Jiao Tong University Medical College, Shanghai, China
Chemical exchange saturation transfer (CEST) imaging shows great potential in clinical application. Separating the nuclear Overhauser enhancement (NOE) and amide proton transfer (APT) effects properly is highly valuable for clinical application of CEST. In this study, the background Z-spectra including only the magnetization transfer and direct saturation effects was fitted by using neural network, then CEST and NOE maps were obtained simultaneously. The reproducibility and feasibility of new method were demonstrated in four healthy volunteers and two patients with ischemic stroke.
|
|||
3709.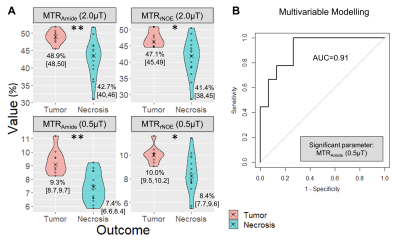 |
Differentiation of Radiation Necrosis from Tumor Progression in Brain Metastasis Treated with Stereotactic Radiosurgery using CEST at 3T
Rachel W Chan1, Hatef Mehrabian1, Hany Soliman2, Hanbo Chen2, Aimee Theriault2, Sten Myrehaug2, Chia-Lin Tseng2, Jay Detsky2, Wilfred W Lam1, Angus Z Lau1,3, Gregory J Czarnota1,2,3, Arjun Sahgal2, and Greg J Stanisz1,3,4
1Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 2Department of Radiation Oncology, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 3Medical Biophysics, Sunnybrook Research Institute, Toronto, ON, Canada, 4Department of Neurosurgery and Pediatric Neurosurgery, Medical University, Lublin, Poland
Stereotactic radiosurgery (SRS) is the standard of care treatment for patients with limited brain metastases; however radiation necrosis can develop. Standard clinical approaches have limited ability in differentiating radiation-induced changes from tumor progression. This work examines the performance of CEST metrics (MTRAmide and MTRrNOE) at 3T for differentiating radiation necrosis from tumor progression, extending a previous study to include higher saturation power (using B1=2.0μT) with added multivariable logistic regression. Results in 24 lesions showed that both saturation powers (0.52μT and 2.0μT) could distinguish tumor progression from radiation necrosis, with the MTRAmide(0.52μT) parameter selected from multivariable modelling with AUC=0.91.
|
|||
3710.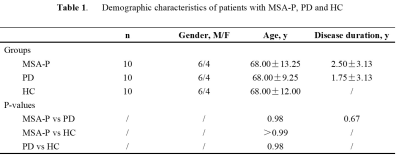 |
Differentiating PD and MSA-P with Neuromelanin and Iron Simultaneously Using a Single 3D Magnetization Transfer Sequence
Yu Liu1, Junchen Li2, Ying Wang3, Naying He1, Zhijia Jin1, Pei Huang4, Shengdi Chen4, Fuhua Yan1, and Ewart Mark Haacke3
1Department of Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2Department of Radiology, Changshu Hospital Affiliated to Nanjing University of Chinese Medicine, Changshu, China, 3Wayne State University, Detroit, MI, United States, 4Department of Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
MSA with a parkinsonian variant (MSA-P) and PD share many common clinical presentations, such as parkinsonism and autonomic failure to mention just a few. The early differential diagnosis of these two disorders has been established more or less empirically despite a lack of a subjective, satisfactory biomarker. Neuroimaging has revolutionized in-vivo visualization of neuromelanin and iron in the substantial nigra (SN), which has been considered as the primary and predominant pathophysiological change in PD patients. We found significant neuromelanin changes in the SN in patients with MSA-P compared with PD and HC.
|
|||
3711.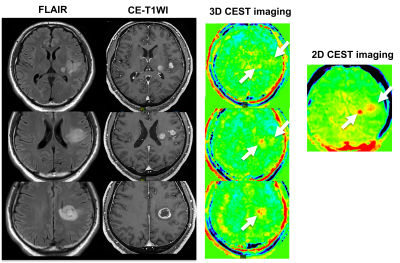 |
Comparison of Capability for Molecular-Based Assessment between 3D Gradient Echo-Based and 2D Spin Echo-Based CEST Imaging for Brain Tumors
Kazuhiro Murayama1, Yoshiharu Ohno2, Masao Yui3, Kaori Yamamoto3, Masato Ikedo3, Satomu Hanamatsu2, Akiyoshi Iwase4, Takashi Fukuba4, and Hiroshi Toyama2
11) Joint Research Laboratory of Advanced Medical Imaging, Fujita Health University School of Medicine, Toyoake, Japan, 2Radiology, Fujita Health University School of Medicine, Toyoake, Japan, 3Canon Medical Systems Corporation, Otawara, Japan, 4Radiology, Fujita Health University Hospital, Toyoake, Japan
No major reports have been evaluated the capability for the molecular-based assessment by 3D chemical exchange saturation transfer (CEST) imaging and compared with 2D CEST imaging. We hypothesized that 3D CEST imaging had equal or better potential for the molecular-based assessment in various brain tumor patients, when compared with 2D CEST imaging. The purpose of this study was to directly compare the capability for molecular-based assessment between 2D and 3D CEST imaging in patients with various brain tumors.
|
|||
3712.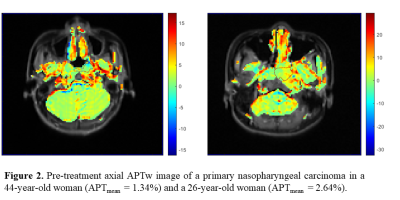 |
Prediction of the response to induction chemotherapy using Amide Proton Transfer MRI in nasopharyngeal carcinoma
Guixiao Xu1, Hui Li1, Yueming Yuan2, Liangru Ke1, Yun He1, Yanlin Zhu1, Liyun Zhen2, Yingyi Huang1, Chuanmiao Xie1, and Yongming Dai2
1Department of Radiology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in Southern China, Guangzhou, China, 2Central Research Institute, United Imaging Healthcare, Shanghai, China
Nasopharyngeal carcinoma (NPC) prevails in Southeast Asia. Induction chemotherapy (IC) is recommended as the effective treatment for patients with NPC. However, not all patients respond well to IC. Pretreatment identification of the non-responders may help to make treatment more personalized. The amide proton transfer (APT) MRI can give contrast due to exchangeable backbone amide protons of endogenous mobile proteins and peptides, and APT value in tumor has been reported higher. Therefore, the aim of this study is to evaluate whether APT value before treatment correlate to IC response in NPC.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.