Oral
Renal
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 5 | 18:00 - 20:00 | Moderators: Durgesh Dwivedi & Steven Sourbron |
 |
0417.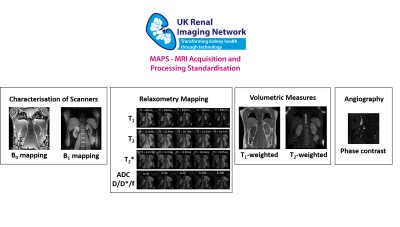 |
Harmonisation of Multiparametric Renal MRI for Multi-Centre Studies
Charlotte E Buchanan1, Hao Li2, Fabio Nery3, Alexander J Daniel1, Joao De Sousa4, Steven Sourbron4, Andrew Priest2,5, David Thomas6,7,8, and Susan T Francis1
1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 3Great Ormond Street Institute of Child Health, University College London, London, United Kingdom, 4University of Sheffield, Sheffield, United Kingdom, 5Department of Radiology, Addenbrooke's Hospital, Cambridge, United Kingdom, 6Neuroradiological Academic Unit, UCL Queen Square Institute of Neurology, UCL, London, United Kingdom, 7Dementia Research Centre, UCL Queen Square Institute of Neurology, UCL, London, United Kingdom, 8Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, UCL, London, United Kingdom
Standardisation and multicentre evaluation of renal MRI measures is crucial for clinical translation. Here we present a multiparametric renal MRI protocol comprising of B0 and B1 mapping, diffusion weighted imaging (DWI), T1, T2 and T2* mapping, phase contrast (PC-MRI) and volumetric T1- and T2-weighted scans that has been harmonised across GE, Philips and Siemens 3T scanners.
|
|
 |
0418.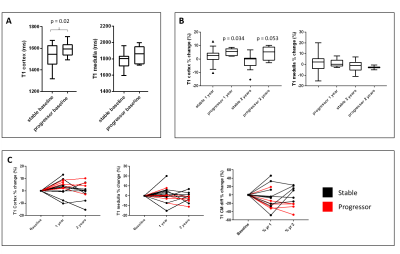 |
Multiparametric Renal MRI in Chronic Kidney Disease: Changes in MRI and Clinical Measures Over Two Years
Charlotte E Buchanan1, Rebecca Noble2, Eleanor Cox1, Huda E Mahmoud2, Isma Kazmi2, Benjamin Prestwich1, Nicholas Selby2, Maarten Taal2, and Susan T Francis1
1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2Centre for Kidney Research and Innovation, University of Nottingham, Derby, United Kingdom
We use multi-parametric renal MRI to assess structural and haemodynamic changes in CKD patients and assess these changes after one and two years to investigate the ability of MRI measures to predict and monitor progression of CKD. At baseline, higher renal cortex T1 and a reduction in renal cortex perfusion are associated with subsequent progression of CKD over 2 years suggesting that these MRI parameters may be predictors of progression. Renal cortex T1 and total kidney volume changed more in ‘progressors’ than in ‘stable’ participants over time compared to baseline suggesting these to be useful MRI measures to monitor progression.
|
|
0419.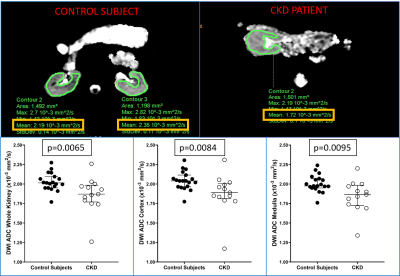 |
Multiparametric Renal MRI in Children and Young Adults: Comparison between Healthy Participants and Patients with Chronic Kidney Disease
Deep B. Gandhi1, Jonathan R. Dillman2, Andrew T. Trout2, Jean A. Tkach2, Prasad Devarajan3, and Stephanie W Benoit4
1Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 2Imaging Research Center, Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 3Department of Nephrology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 4Department off Nephrology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
Multiparametric renal MRI might be used as a non-invasive biomarker of pediatric chronic kidney disease (CKD). 20 pediatric and young adult healthy controls and 12 patients with CKD underwent quantitative renal MRI consisting of MR elastography (MRE), T1 mapping, T2 mapping, and diffusion-weighted imaging (DWI). Whole kidney and cortical T1 values were greater in patients than healthy controls (p=0.018 and p<0.0001, respectively), whereas whole kidney, cortical, and medullary DWI ADC values were lower in patients than healthy controls (p=0.017, whole kidney). No differences in T2 or stiffness measurements between the two groups were observed.
|
||
 |
0420.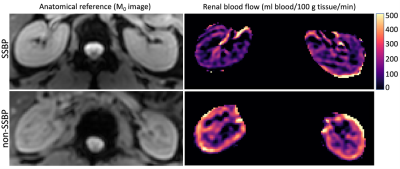 |
Renal perfusion imaging with free-breathing pCASL MRI in persons with salt-sensitive blood pressure
Michael Pridmore1, Maria Garza1, Laura Jones2, Cassandra Reynolds2, Deepak Gupta2, Manus Donahue1, and Rachelle Crescenzi1
1Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 2Cardiovascular Medicine, Vanderbilt University Medical Center, Nashville, TN, United States
Salt sensitive blood pressure is a major independent risk factor for cardiovascular disease, with estimated 50% prevalence in adults for which no imaging biomarker exists. We evaluated persons for salt sensitivity and applied a quantitative magnetic resonance imaging strategy in the kidneys for measuring renal blood flow using free-breathing pseudo-continuous arterial spin labeling. Group comparisons showed renal blood flow is reduced in subjects with salt-sensitivity, which may be linked to renal mechanisms of sodium handling. Additionally, image acquisition protocols were compared between 20x and 4x acquisitions, revealing 4x acquisitions were robust to motion and favored a clinically feasible scan time.
|
|
0421.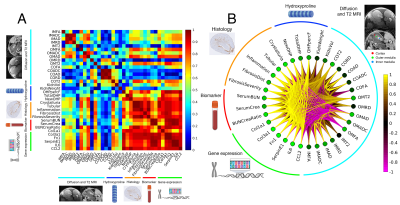 |
Imaging renal fibrosis in an oxalate induced chronic kidney disease model
Luke Xie1, Aaron K Wong2, Rohan S. Virgincar1, Patrick Caplazi3, Vineela D. Gandham1, Alex J. De Crespigny4, Robby M. Weimer1, and Hans D. Brightbill2
1Biomedical Imaging, Genentech, South San Francisco, CA, United States, 2Translational Immunology, Genentech, South San Francisco, CA, United States, 3Pathology, Genentech, South San Francisco, CA, United States, 4Clinical Imaging Group, Genentech, South San Francisco, CA, United States
Chronic kidney disease (CKD) is a significant global health problem with fibrosis being a common pathway of disease progression. While MRI is sensitive to fibrosis, the relationship to ultrastructural underpinnings is not well understood. In this study, we evaluate an oxalate induced CKD model and determine the correlation of MRI metrics with high-resolution terminal endpoints. We find that FA and AD in the medulla are most correlated with fibrosis pathologies, new hydroxyproline, and inflammatory and fibrotic gene expression. These results show that MRI can detect fibrosis and that the signal change is related to interstitial fibrosis and nephron ultrastructure.
|
||
 |
0422.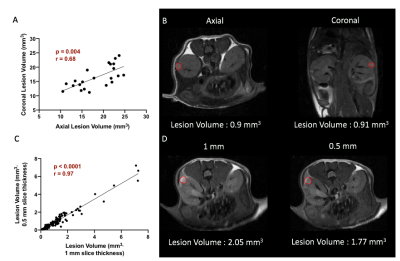 |
MR imaging of tuberous sclerosis complex in kidneys
Shubhangi Agarwal1, Emilie Decavel-Bueff1, Yung-Hua Wang1, Hecong Qin1, Romelyn Delos Santos1, Michael Evans1, and Renuka Sriram1
1Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States
There is a critical need for the development of non-invasive imaging strategies for tuberous sclerosis (TSC) derived tumor lesions in order to monitor the progression and therapeutic efficacy of this multi-system genetic disease. In this study, we investigated the ability of multiparametric 1H MRI (morphological T2-weighted and functional diffusion-weighted) to meet this need in a preclinical murine model of TSC treated with everolimus. Results indicated that proton MR imaging was able to capture the changes in cellularity and tumor size, post treatment.
|
|
0423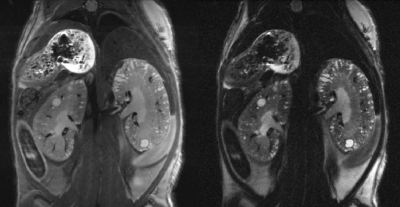 |
High-resolution kidney MRI in mice for longitudinal tracking of kidney volume and cyst burden Video Permission Withheld
Florian Schmid1, Geogios Koukos1, Yi Liu1, Matt Sooknah1, Sandip Chatterjee1, Adam Freund1, and Johannes Riegler1
1Calico Life Sciences LLC, South San Francisco, CA, United States
We present an improved imaging and data analysis strategy for kidney MRI in mouse models of polycystic kidney disease. By registering multiple high resolution datasets at lower SNR, slow movement is compensated for and high resolution datasets with high quality and fine detail are achieved, allowing for detection and longitudinal tracking of kidney volume as well as cyst number and size. We established automatic cyst detection and are developing automatic kidney segmentation, for accurate and reliable assessment of polycystic kidney disease in mouse models.
|
||
0424.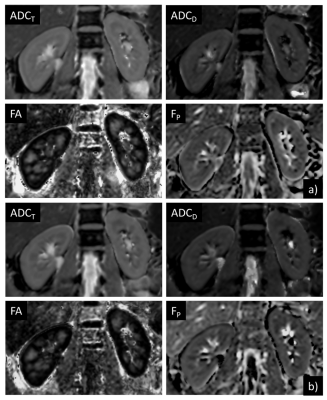 |
Motion-insensitive DTI of Kidney using Prospective Acquisition Motion Correction Triggering
Arun Joseph1,2,3, Laila-Yasmin Mani4, Tom Hilbert5,6,7, Thomas Benkert8, Tobias Kober5,6,7, Bruno Vogt4, and Peter Vermathen3
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 2Translational Imaging Center, Sitem-Insel, Bern, Switzerland, 3Departments of Radiology and Biomedical Research, University of Bern, Bern, Switzerland, 4Department of Nephrology and Hypertension, University Hospital Bern, Inselspital, Bern, Switzerland, 5Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 6Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 7LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 8Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Diffusion tensor imaging (DTI) of the kidney provides important functional information such as diffusion and micro-perfusion of the tissue and additionally estimates anisotropic diffusion of water in renal tubuli. However, these measurements are highly sensitive to respiration-induced motion artifacts which bias the obtained functional information. Here, we propose to use prospective acquisition motion correction (PACE) in combination with free-breathing acquisitions for motion-insensitive diffusion measurements of the kidney. A preliminary qualitative and quantitative validation is performed on healthy subjects comparing results from conventional respiratory-triggered to PACE-triggered DTI.
|
||
0425.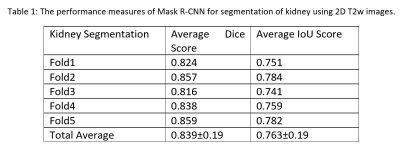 |
Mask R-CNN for Segmentation of Kidneys in Magnetic Resonance Imaging
Manu Goyal1, Junyu Guo1, Lauren Hinojosa1, Keith Hulsey1, and Ivan Pedrosa1
1Radiology, UT Southwestern Medical Center, Dallas, TX, United States
Automated segmentation of kidneys in Magnetic Resonance Imaging (MRI) exams are important for enabling radiomics and machine learning analysis of renal disease. In this work, we propose to use a deep learning method called Mask R-CNN for the segmentation of kidneys in 2D coronal T2W FSE images of 94 MRI exams. With 5-fold cross-validation data, the Mask R-CNN is trained and validated on 66 and 9 MRI exams and then evaluated on the remaining 19 exams. Our proposed method achieved an average dice score of 0.839 and an average IoU of 0.763.
|
||
 |
0426.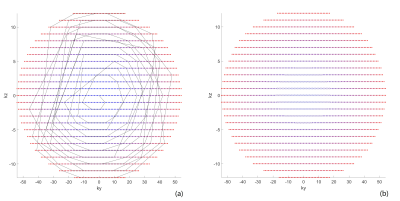 |
Volumetric Renal ASL MRI using 3D TSE Cartesian Acquisition with Variable Density Sampling (VD-CASPR)
Yiming Wang1, Limin Zhou1, Ivan Pedrosa1,2,3, and Ananth J. Madhuranthakam1,2
1Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 2Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 3Urology, UT Southwestern Medical Center, Dallas, TX, United States
3D Cartesian turbo spin echo (TSE) is a promising acquisition method for renal ASL because of its improved SNR and compatibility with optimal background suppression (BGS). However, 3D Cartesian TSE with single average can limit its SNR and robustness. In this study, we applied a variable density sampling approach to renal ASL imaging, which acquires the center of the k-space with higher signal averages and improves SNR and robustness without significantly prolonging scan time. We combined this with partial k-space acquired M0 images to compensate for increased scan time, but without compromising perfusion quantification.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.