Digital Poster
Acquisition & Analysis Methods for Task-Based fMRI
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
2111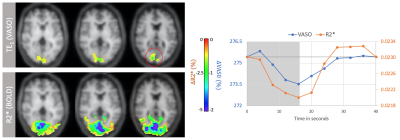 |
13 | CBV-Based fMRI at 3T with SS-SI-VASO: Multi-Echo DEPICTING vs Multi-Echo EPI
Ratnamanjuri Devi1, Toralf Mildner1, Torsten Schlumm1, and Harald E. Möller1
1Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
ME-DEPICTING, a 2D multi-echo readout achieving short echo times was combined with SS-SI-VASO for simultaneous CBV and BOLD measurements at 3T. Results were compared with a traditional ME-EPI readout. Uncorrected time courses of the first echo of ME-DEPICTING demonstrated high sensitivity to functional CBV changes without further BOLD correction, while time courses of S0 and R2* of the multi-echo fit proved almost equivalent for both readouts. Remarkably, a TE dependence was observed in the BOLD-corrected VASO signals obtained by dynamic division with the corresponding control images of VASO indicating a more reliable CBV measure from the S0 fit.
|
||
2112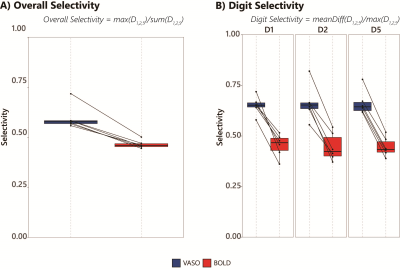 |
14 | Improved digit selectivity using VASO-CBV in 7T digit motor mapping
Icaro A.F. Oliveira1,2, Jeroen C.W. Siero1,3, Serge O. Dumoulin1,2,4, and Wietske van der Zwaag1
1Spinoza Centre for Neuroimaging, Amsterdam, Netherlands, 2Experimental and Applied Psychology, VU University, Amsterdam, Netherlands, 3Radiology, University Medical Centre Utrecht, Utrecht, Netherlands, 4Experimental Psychology, Helmholtz Institute, Utrecht University, Utrecht, Netherlands
We used sub-millimeter resolution VASO fMRI at 7T to simultaneously map CBV-VASO and BOLD activation in the sensorimotor and somatosensory cortex during a finger digit mapping task. We reliably showed digit representation maps and quantified digit selectivity for both VASO-CBV and BOLD activations. VASO-CBV demonstrated a higher digit selectivity, confirming the higher spatial specificity of VASO-CBV.
|
||
2113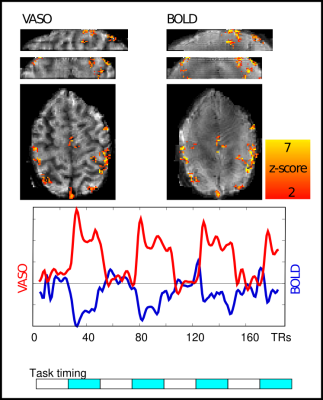 |
15 | Combining the benefits of 3D acquisitions and spiral readouts in VASO fMRI
Alejandro Monreal1, Ruoyun Emily Ma1, Renzo Huber1, Denizhan Kurban1, Nicolas Boulant2, and Benedikt A Poser1
1Maastricht University, Maastricht, Netherlands, 2CEA NeuroSpin, Gif-sur-Yvette, France VASO fMRI can provide beneficial localization specificity and quantifiability compared to the commonly used BOLD contrast. Previous work has also shown the benefits of using spiral readouts compared to Cartesian. In this work, we explore the benefits of 3D stack of spirals readouts and compare it with the current state of the art 3D EPI readouts for VASO fMRI. The sequence implementation is done using Pulseq, images were reconstructed using gpuNUFFT; functional analysis with an openly available pipeline. We find that a tSNR efficiency improvement of a factor of 2.5 over EPI is achieved using the proposed spiral implementation. |
||
2114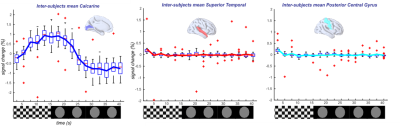 |
16 | A novel free-running framework for Blood Oxygen Level Dependent functional MRI
Benedetta Franceschiello1,2, Simone Rumac1, Tom Hilbert1,3,4, Christopher W. Roy1, Giulio Degano5, Anna Gaglianese1, Jerome Yerly1,2, Matthias Stuber1,2, Tobias Kober1,3,4, Ruud B. van Heeswijk1, Micah M. Murray1,2,6,7, and Eleonora Fornari1,2,7
1Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 2CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 3Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 4LTS5, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 5Department of Psychology, Faculty of Psychology and Educational Sciences, University of Geneva, Geneva, Switzerland, 6Department of Hearing and Speech Sciences, Vanderbilt University, Nashville, TN, United States, 7These authors provided equal last-authorship contribution, Lausanne, Switzerland
We demonstrate the efficacy of a new free-running framework for quantitatively measuring blood-oxygen-level-dependent functional MRI (BOLD-fMRI) signals. Whole-brain data were acquired uninterruptedly during a blocked-design ON/OFF visual paradigm (checkerboard vs. grey image). 5-D volumes were reconstructed (i.e. the three spatial dimensions (x,y,z), the trial dimension (N=33), and the ON vs. OFF dimension (N=2)). BOLD-activated regions were measured as the difference between volumes reconstructed from all read-outs acquired during the ON vs. OFF condition, rather than resulting from a canonical, model-based, GLM analysis. The framework enables high temporal T2* k-space sampling and opens up new horizons for BOLD-fMRI.
|
||
2115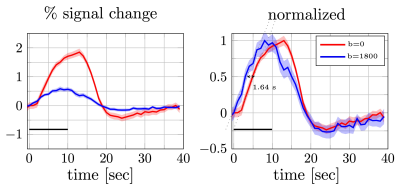 |
17 | Detection of fast responses in diffusion fMRI of the human visual cortex through reduced vascular contamination
Shota Hodono1,2, Jonathan R Polimeni3,4, and Martijn A Cloos1,2
1Centre for Advanced Imaging,The University of Queensland, Brisbane, Australia, 2ARC Training Centre for Innovation in Biomedical Imaging Technology, The University of Queensland, Brisbane, Australia, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Harvard Medical School, Massachusetts General Hospital, Charlestown, MA, United States, 4Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
A key feature of the diffusion-weighted fMRI (
|
||
2116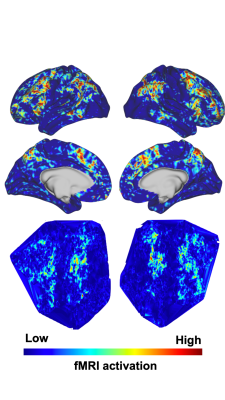 |
18 | Convolutional neural network predicts task fMRI working memory scores and enables further understanding of working memory network
Mario Serrano-Sosa1, Jared X. Van Snellenberg1,2,3, and Chuan Huang1,2,4
1Biomedical Engineering, Stony Brook University, Stony Brook, NY, United States, 2Psychiatry, Stony Brook Medicine, Stony Brook, NY, United States, 3Psychology, Stony Brook University, Stony Brook, NY, United States, 4Radiology, Stony Brook Medicine, Stony Brook, NY, United States
Interpretable Deep Learning(DL) models are the next step in establishing DL prediction models as accepted tools that provide researchers with data-driven methods to further understand neuroimaging data. In this work, we developed two interpretable DL models to predict Working Memory(WM) scores from task fMRI data to assess neural circuitry pertaining to WM; wherein a traditional Convolutional Neural Network(CNN)(1-3) contained fMRI activation data from cortical vertices as a single image, and the second contained cortical activation data from both hemispheres as separate channels. Overall, the interpretable DL model provided high quality saliency maps potentially displaying novel regions pertaining to WM.
|
||
2117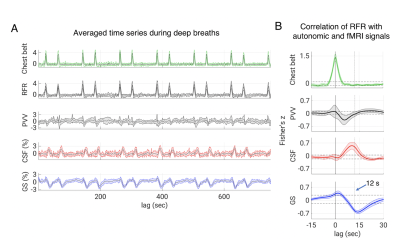 |
19 | Effects of Autonomic Activity on Brain Fluid Pulsations
Pinar S Özbay1, Dante Picchioni1, Hendrik Mandelkow1, Jacco A de Zwart1, Yicun Wang1, Peter van Gelderen1, and Jeff Duyn1
1NINDS, NIH, Bethesda, MD, United States
According to recent research, slow waves may increase metabolic waste clearance during sleep, possibly through cerebrospinal fluid (CSF) pulsatile movement. Pulsations can also be caused by direct pressure effects from the cardiac and respiratory cycles, such as deep breaths. To examine this possibility, we designed a cued deep breathing experiment, which was previously demonstrated to result in widespread fMRI global signal reductions. In this work, our findings point to an alternative pathway for the creation of CSF pulsations that relies on autonomic changes rather than sleep.
|
||
2118 |
20 | Temporal properties of fast BOLD fMRI responses in veins and parenchyma
Daniel E. P. Gomez1,2,3, Nina E. Fultz1,2,4, Jonathan R. Polimeni1,3,5, and Laura D. Lewis2
1Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Biomedical Engineering, Boston University, Boston, MA, United States, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Department of Neuroscience, University of Copenhagen, Copenhagen, Denmark, 5Harvard-MIT Division of Life Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Blood-oxygen-level-dependent (BOLD) signals can produce much faster (>0.2Hz) responses than predicted by canonical hemodynamic response models. The timing of hemodynamic responses depends on vascular anatomy, and whether these fast responses differ in veins and parenchyma is unknown. Linear models predict that with faster stimuli, venous responses should attenuate more than the parenchyma. We tested this hypothesis by imaging visual responses at high spatial resolution with 7T fMRI. We found that both veins and parenchyma produce larger fMRI responses to fast stimuli than predicted by linear models, suggesting that faster stimuli produce narrower hemodynamic responses in both compartments.
|
||
2119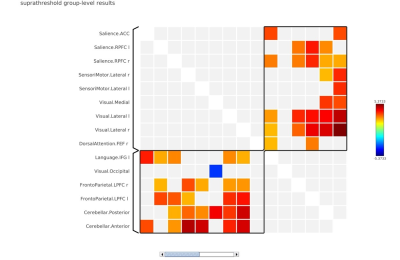 |
21 | Understanding the effect of noise on cognition using attention framework Navon task
S Senthil Kumaran1, Himanshu Singh1, and A Ankeeta1
1Department of NMR and MRI Facility, All India Institute of Medical Sciences, New Delhi, India
Cognitive studies using functional imaging are inherently associated with nonstimulant noise in MRI. Dissociating the noise aspect in functional imaging from cognitive traits is crucial in delineating auditory cognition. This study explores the association of noise in auditory cognition studies. we designed a functional Navon task paradigm with introduction of pink noise characteristics modelled as part of its memory phase. Variation of noise induced the cognitive masking associated with characteristics of stimuli across the local and global suppression.
|
||
2120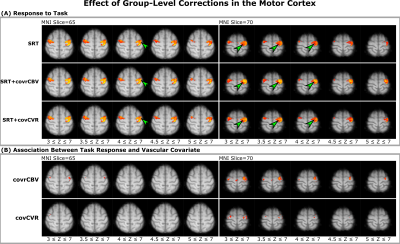 |
22 | Reducing Between-Subject Variability in Stimulus-Evoked BOLD fMRI Using Breath Hold-Derived Vascular Covariates
Emma Biondetti1,2, Antonio Maria Chiarelli1,2, Ilona Lipp3,4, Rachael Stickland3,5, Alessandro Villani1,2, Eleonora Patitucci3, Valentina Tomassini1,2,6,7,8, and Richard Wise1,2
1Department of Neuroscience, Imaging, and Clinical Sciences, D'Annunzio University of Chieti-Pescara, Chieti, Italy, 2Institute for Advanced Biomedical Technologies, D'Annunzio University of Chieti-Pescara, Chieti, Italy, 3Cardiff University Brain Research Imaging Centre (CUBRIC), School of Psychology, Cardiff, United Kingdom, 4Department of Neurophysics, Max Planck Institute for Human Cognitive & Brain Sciences, Leipzig, Germany, 5Physical Therapy and Human Movement Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 6MS Centre, Neurology Unit, SS. Annunziata University Hospital, Chieti, Italy, 7Division of Psychological Medicine and Clinical Neurosciences, School of Medicine, Cardiff University, Cardiff, United Kingdom, 8Helen Durham Centre for Neuroinflammation, University Hospital of Wales, Cardiff, United Kingdom
The representation of changes in neural activity by blood oxygenation level-dependent (BOLD) fMRI is affected by vascular properties including cerebral blood volume (CBV) and cerebral vascular reactivity (CVR). Here, we investigate these factors using a breath-hold BOLD fMRI experiment. We show that a group-level correction of the stimulus-evoked BOLD fMRI signal by these vascular properties reduces between-subject variability and improves sensitivity to the task response. Notably, correcting with a marker of relative CBV does not require individual recordings of exhaled carbon dioxide (in contrast with CVR) and resulted in the highest increase in sensitivity to the task.
|
||
2121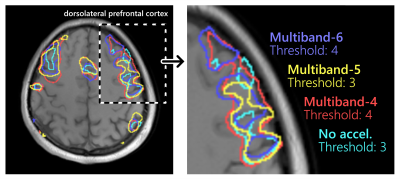 |
23 | Comparison of different multiband acceleration factors on rhyming-task fMRI on a compact 3T scanner
Zaki Ahmed1, Kirk M Welker1, Maria A Halverson1, Nolan K Meyer1, Daehun Kang1, Myung-Ho In1, Erin M Gray1, David F Black1, Joshua D Trzasko1, John Huston III1, Matt A Bernstein1, and Yunhong Shu1
1Department of Radiology, Mayo Clinic, Rochester, MN, United States
Multiband acquisition can improve the temporal resolution of task-based fMRI at the cost of increased noise and artifacts. The optimal multiband acceleration depends on the task being studied, specific imaging protocol, and hardware involved. In the current work, multiband acceleration factors of 4, 5, and 6 were compared for a rhyming-task fMRI study using a 32-channel head coil on a compact 3T scanner with high-performance gradients. Multiband factors of 4 and 6 provided significant improvement over no acceleration, whereas a multiband factor of 5 was inconsistent and did not provide a significant improvement in half of the volunteer cohort.
|
||
2122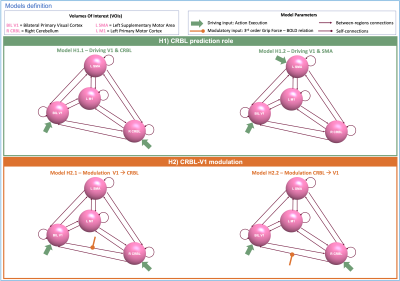 |
24 | The role of Cerebellum in a visuomotor task: A Dynamic Causal Modeling approach
Roberta Maria Lorenzi1, Adnan Alahmadi2,3, Letizia Casiraghi1,4, Anita Monteverdi1,5, Egidio D'Angelo1,5, Fulvia Palesi1, and Claudia AM Gandini Wheeler-Kingshott1,3,5
1Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy, 2Department of Diagnostic Radiology, College of Applied medical sciences, King Abdulaziz University, Jeddah, Saudi Arabia, 3NMR Research Unit, Queen Square Multiple Sclerosis Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, UCL, London, United Kingdom, 4Department of Mental Health and Dependence, ASST of Pavia, Pavia, Italy, 5Brain Connectivity Center, IRCCS Mondino Foundation, Pavia, Italy
Dynamic Causal Modelling(DCM) is a framework enabling the quantification of the causal relationship between functionally connected brain regions. Here we included the cerebellum in a visuomotor network to investigate its role in motion prediction and how different Grip-Force levels modulate the effective connectivity between cerebellum and primary visual cortex. We estimated different DCMs models and we used Bayesian Models Selection to assess the best model at group-level. This study paves the way for investigating the source of BOLD non-linearities when applying different grip-forces in terms of modulations of the cerebro-cerebellar connections.
|
||
2123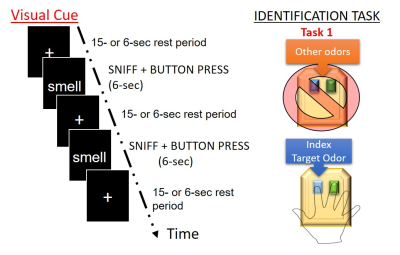 |
25 | Olfactory Signal processing in functional MRI oddball task
Prasanna Karunanayaka1, Andréa Hobkirk2, Jianli Wang1, Rommy Elyan1, Lauren Spreen3, Ran Pang4, Samgan Kanekar1, and Qing Yang4
1Department of Radiology, Pennsylvania State University College of Medicine, Hershey, PA, United States, 2Department of Psychiatry, Pennsylvania State University College of Medicine, Hershey, PA, United States, 3Department of Molecular Biology, Pennsylvania State University College of Medicine, Hershey, PA, United States, 4Department of Neurosurgery, Pennsylvania State University College of Medicine, Hershey, PA, United States
Odor-identification is thought to be subserved by a distributed brain network that includes both the medial and inferior temporal lobes, still, its neural substrate remains poorly understood. Odor identification deficits are one of the hallmark symptoms of early Alzheimer’s disease (AD). Here, we propose the development of an oddball olfactory fMRI paradigm to establish the neural basis of odor-identification in healthy subjects. This task may provide a basis for establishing relationships between olfactory deficits, neurodegeneration, and memory impairment for current AD research.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.