Digital Poster
MT & CEST
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
2705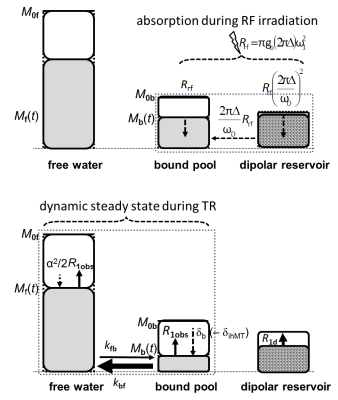 |
69 | Understanding inhomogeneous MT (ihMT) in multi-parameter mapping of human brain: Towards larger ihMT, higher resolution, and influence of T1d
Gunther Helms1,2, Lenka Vaculčiaková2, Kerrin J Pine2, Harald E Möller3, and Nikolaus Weiskopf2,4
1Clinical Sciences, Medical Radiation Physics, Lund University, Lund, Sweden, 2Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3NMR Methods & Development Group, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 4Felix Bloch Institute for Solid State Physics, Leipzig University, Leipzig, Germany
Inhomogenous Magnetization Transfer (ihMT), the differential response to irradiation at single and dual frequency offsets, is more complex than MT approaches. Expressing ihMT in terms of MT-saturation (ihMTsat) is a first step to quantification as it corrects for underlying T1 and B1+. Larger ihMTsat was observed for smaller frequency offsets, which is explained using MTsat as proxy for bound pool saturation. For longer TR, ihMTsat increased faster than MTsat indicating recovery of dipolar order as ihMTsat increased super-linearly to 1.1pu in WM and 0.2pu in GM at TR=52ms. ihMTsat mapping in vivo was performed at 1.3mm isotropic resolution.
|
||
2706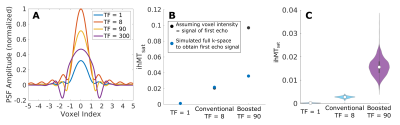 |
70 | Acquisition optimization for cortical ihMTsat imaging
Christopher D Rowley1,2, Ilana R. Leppert1, Jennifer S.W. Campbell1, G Bruce Pike3, and Christine L Tardif1,2,4
1McConnell Brain Imaging Centre, McGill University, Montreal, QC, Canada, 2Neurology and Neurosurgery, McGill University, Montreal, QC, Canada, 3Hotchkiss Brain Institute and Departments of Radiology and Clinical Neuroscience, University of Calgary, Calgary, AB, Canada, 4Biomedical Engineering, McGill University, Montreal, QC, Canada Sequence simulations are used to compare different conventional and boosted saturation schemes and imaging protocols for the purpose of high-resolution ihMTsat cortical imaging. The simulations are constrained to a maximum of four minutes per volume. We find an optimal region for conventional saturation that produces the greatest ihMTsat. For the boosted approach, larger ihMTsat values are found with respect to the conventional approach, and ihMTsat increases with longer TRs. However, the practical utility of the boosted approach for cortical imaging is hampered by a wider PSF associated with higher turbo factors. Simulated ihMTsat findings are supported by in vivo data. |
||
2707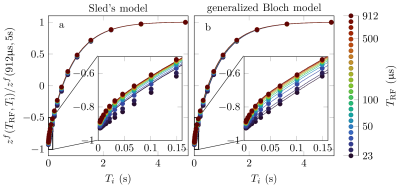 |
71 | Generalized Bloch model: a theory for pulsed magnetization transfer
Jakob Assländer1,2, Cem Gultekin3, Sebastian Flassbeck1,2, Steffen J Glaser4, and Daniel K Sodickson1,2
1Center for Biomedical Imaging, New York University School of Medicine, New York, NY, United States, 2Center for Advanced Imaging Innovation and Research, New York University School of Medicine, New York, NY, United States, 3Courant Institute of Mathematical Sciences, New York University, New York, NY, United States, 4Department of Chemistry, Technische Universität München, München, Germany
We introduce a classical model to describe magnetization transfer (MT). Like a quantum-mechanical description of spin dynamics and like the original Bloch equations, but unlike existing MT models, the proposed model is based on the algebra of angular momentum in the sense that it explicitly models the rotations induced by radio-frequency (RF) pulses. It unifies the original Bloch model, Henkelman's steady-state theory for magnetization transfer, and the commonly assumed rotation induced by hard, i.e. short, pulses, and describes experimental data better than previous models.
|
||
2708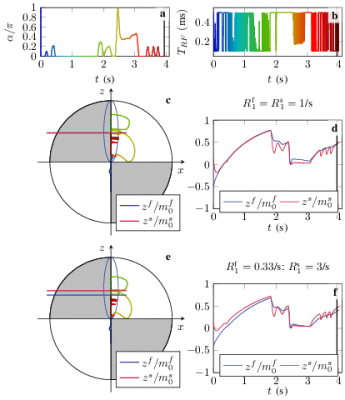 |
72 | Quantitative magnetization transfer: Estimation of the Semi-Solid Spin Pool's T1
Sebastian Flassbeck1,2 and Jakob Assländer1,2
1Center for Biomedical Imaging, New York University School of Medicine, New York, NY, United States, 2Center for Advanced Imaging Innovation and Research, New York University School of Medicine, New York, NY, United States
Most magnetization transfer experiments are insensitive to the longitudinal relaxation rate of the invisible semi-solid spin pool and it has become common practice to heuristically assume 1/second. This assumption has, however, been challenged recently. Because this parameter is notoriously hard to estimate, previous studies have relied on the analysis of large white matter regions or the entire brain. Here, we explore the ability to estimate this value on a voxel-by-voxel basis with an MR-Fingerprinting-like pulse sequence.
|
||
2709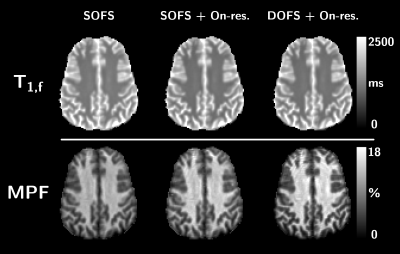 |
73 | Quantitative Magnetization Transfer parametric mapping unbiased by on-resonance saturation and dipolar order contributions
Lucas Soustelle1,2, Thomas Troalen3, Andreea Hertanu1,2, Maxime Guye1,2, Jean-Philippe Ranjeva1,2, Gopal Varma4, David C. Alsop4, Olivier M. Girard1,2, and Guillaume Duhamel1,2
1Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3Siemens Healthcare SAS, Saint-Denis, France, Paris, France, 4Division of MR Research, Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Two major biases in the current qMT methodology arise from i) on-resonance saturation effects induced by readout pulses used in variable flip angle SPGR experiments, and ii) dipolar order effects induced by single-frequency off-resonance saturation pulses leading to a reduced saturation efficiency. In this work, we evaluate these two biases by performing experiments and analyses by accounting for on-resonance saturation in the qMT model and using simultaneous symmetric dual-offset frequency saturation pulses to cancel out dipolar order effects. Results show improvement in the fitting procedure and more accurate estimation of T1,f and MPF values in white and grey matter structures.
|
||
2710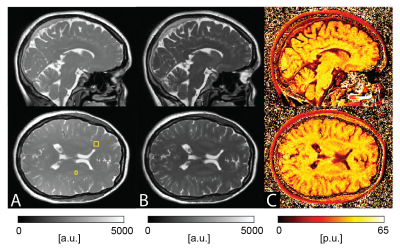 |
74 | On the Prospects of Magnetization Transfer Imaging at 0.55T
Roya Afshari1,2, Grzegorz Bauman1,2, and Oliver Bieri1,2
1Division of Radiological Physics, Department of Radiology, University Hospital Basel, Basel, Switzerland, 2Department of Biomedical Engineering, University of Basel, Basel, Switzerland
Magnetization transfer (MT) imaging has been extensively used to explore microstructural changes in the brain at high fields. In this work, we explore the potential of a 3D half-radial dual-echo balanced steady-state free precession (bSSFP) sequence for fast whole-brain magnetization transfer ratio (MTR) imaging at low-field strength. Our work indicates superiority of MT-sensitized bSSFP against conventional MT-prepared spoiled gradient echo (SPGR) in terms of MT contrast and resolution within similar scan time. In conclusion, MTR imaging with bSSFP offers excellent prospects for broad clinical translation and application at low fields.
|
||
2711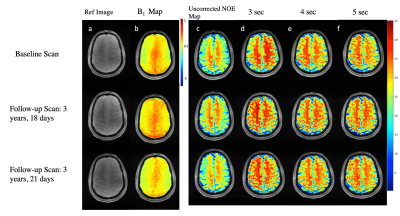 |
75 | Reproducibility of 3D NOE-MTR in a in vivo healthy human brain at 7T
Blake Benyard1, Ravi Prakash Reddy Nanga1, Neil Wilson1, Deepa Thakuri1, Abigail Cember1, and Ravindeer Reddy1
1CAMIPM, Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States
Nuclear Overhauser Effect (NOE) is an emerging technique to study mobile macromolecules such as lipids in the gray (GM) and white matter (WM) of the brain. In this work, we optimized the saturation pulse parameters of NOE Magnetization Transfer Ratio (MTR) MRI and investigated its reproducibility on a healthy human volunteer at 7 Telsa. We found that NOE-MTR of GM and WM regions of the brain was highly reproducible (COV<10%) over a three year time span. In addition, a saturation length of 3 to 4 sec provided optimal NOE-MTR contrast from both GM and WM regions of the brain.
|
||
2712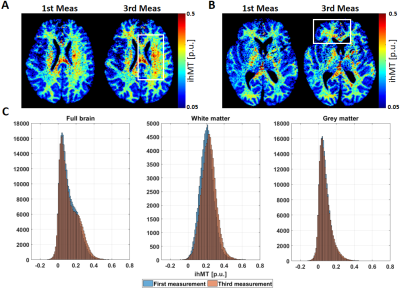 |
76 | Effects of temperature in the estimation of inhomogeneous magnetic transfer (ihMT) in post-mortem human brain
Francisco J. Fritz1, Gunther Helms2,3, Lenka Vaculčiaková3, Nikolaus Weiskopf3,4, and Siawoosh Mohammadi1,3
1Institut für Systemische Neurowissenschaften, Universitätklinikum Hamburg-Eppendorf, Hamburg, Germany, 2Medical Radiation Physics, IKVL, Lund University, Lund, Sweden, 3Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 4Felix Bloch Institute for Solid State Physics, Faculty of Physics and Earth Sciences, Leipzig University, Leipzig, Germany
Inhomogeneous magnetic transfer (ihMT) is more sensitive to myelin macromolecules than standard MT proxies. Measuring ihMT in the multi-parameter mapping protocol allows calculating ihMT from MT saturation (MTsat) maps and thus inherently correct for the undesired dependencies on flip angle and the longitudinal relaxation rate. Further validation of this new ihMT metric requires measurement of MPM-based ihMT of human post-mortem material. Here, we showed that ihMT of a whole human post-mortem brain is feasible but can lead to temperature increase in the specimen, which is particularly pronounced in white matter.
|
||
2713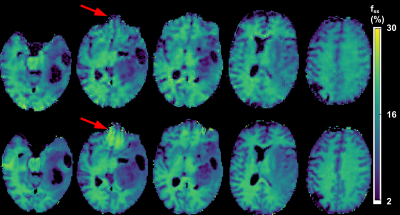 |
77 | Acceleration of Quantitative Semisolid MT/CEST Imaging using a Generative Adversarial Network (GAN-CEST)
Jonah P. W. Weigand1, Maria Sedykh2, Kai Herz3,4, Jaume Coll-Font1,5, Christopher Nguyen1,5,6, Moritz Zaiss2,3, Christian T. Farrar1, and Or Perlman1
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Department of Neuroradiology, Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), University Hospital Erlangen, Erlangen, Germany, 3Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tubingen, Germany, 4Department of Biomedical Magnetic Resonance, University of Tübingen, Tübingen, Germany, 5Cardiovascular Research Center, Cardiology Division, Massachusetts General Hospital, Charlestown, MA, United States, 6Health Science Technology, Harvard-MIT, Cambridge, MA, United States
Quantitative metabolite concentration and pH biomarker maps, as provided by semisolid MT/CEST-MR-Fingerprinting (MRF), constitute a useful means for determining the molecular origin of pathology. However, the lengthy dictionary generation time and the prolonged 3D acquisition time may hinder clinical dissemination. Here, we developed a generative adversarial network (GAN), aimed to drastically shorten the 3D semisolid MT/CEST-MRF acquisition time and circumvent the need for dictionary generation. In-vitro and in-vivo experiments in 4 volunteers and a patient were conducted at 3 different sites using 3 different scanner models, showing substantial reduction in scan time, while retaining a good agreement with ground-truth reference.
|
||
2714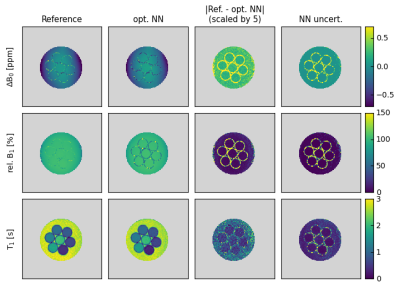 |
78 | Simultaneous Mapping of B0, B1 and T1 Using a CEST-like Pulse Sequence and Neural Network Analysis
Patrick Schuenke1, Kerstin Heinecke1, George Henrik Narvaez1, Moritz Zaiss2, and Christoph Kolbitsch1
1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Department of Neuroradiology, Friedrich- Alexander Universität Erlangen- Nürnberg, University Hospital Erlangen, Erlangen, Germany
Obtaining isolated effects in Chemical Exchange Saturation Transfer (CEST) MRI requires the correction for the influence of B0- and B1-inhomogeneities and the T1 relaxation time during post-processing. We present an extended approach for simultaneous mapping of B0, B1 and T1 using a CEST-like pulse sequence and a regularized neural network-based analysis that provides additional uncertainty estimations. We demonstrate the in vitro and in vivo applicability of the proposed approach, which is trained using simulated data and confirm its accordance with established reference methods.
|
||
2715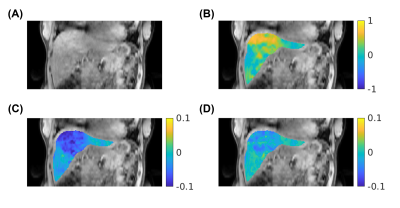 |
79 | Free-breathing 3D CEST MRI of Human Liver at 3T Using MR Multitasking
Pei Han1,2, Tianle Cao1,2, Karandeep Cheema1,2, Hsu-Lei Lee1, Fei Han3, Nan Wang4, Hui Han1, Yibin Xie1, Anthony G. Christodoulou1,2, and Debiao Li1,2
1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, UCLA, Los Angeles, CA, United States, 3Siemens Healthineers, Los Angeles, CA, United States, 4Department of Radiology, Stanford University, Stanford, CA, United States
We developed a 3D abdominal CEST MRI technique at 3T using MR Multitasking, which enables entire-liver coverage with free-breathing acquisition. By exploiting the correlation among images throughout the spatial, time, frequency offset, and respiration dimensions, the low-rank tensor framework shows the possibility to acquire the whole Z-spectrum of 53 frequency offsets within 11 min.
|
||
2716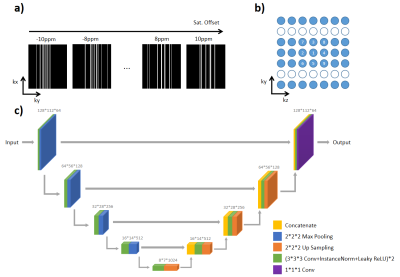 |
80 | FAR-CEST: Fast Acquisition and Reconstruction for Chemical Exchange Saturation Transfer (CEST) imaging using a Deep-Learning Approach
Chuyu Liu1, Zhensen Chen2, Yibing Chen3, Xubin Chai1, Zhongsen Li1, and Xiaolei Song1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 2Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 3Xi’an Key Lab of Radiomics and Intelligent Perception, School of Information Sciences and Technology, Northwest University, Xi'an, China
We developed a Fast Acquisition and Reconstruction CEST (FAR-CEST) method at 3T human scanners, based on a deep learning approach. A 10X accelerated acquisition was achieved, which under-sampled K-space using a randomized Cartesian pattern of variable density. To fully utilize the correlation among saturation offset dimension, especially to compensate for sparsely-sampled K-space edge, a 3D-Res-Unet model was trained for reconstruction. Results on healthy adult brain suggested that FAR-CEST can produce high quality saturation-weighted images and Z-spectra,but the CEST contrast slightly altered. The highly-acceleration feature of FAR-CEST has been initially validated, yet still require improvement on reconstruction accuracy.
|
||
2717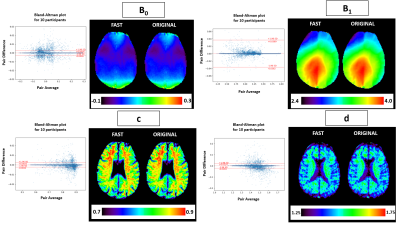 |
81 | Fast WASAB1 post-processing for simultaneous B0 and B1 mapping for CEST imaging use in clinical routine
Christos Papageorgakis1, Eleni Firippi1, Benoit Gy1, Timothé Boutelier1, Ibrahim Khormi2,3,4, Oun Al-iedani2,3, Bryan Paton3,5, Jeannette Lechner-Scott 3,6,7, Amir Fazlollahi8, Anne-Louise Ponsonby9,10, Patrick Liebig11, Saadallah Ramadan2,3, Moritz Zaiss12, and Stefano Casagranda1
1Department of Research & Innovation, Olea Medical, La Ciotat, France, 2School of Health Sciences, College of Health, Medicine and Wellbeing,, University of Newcastle, Newcastle, Australia, 3Hunter Medical Research Institute, Newcastle, Australia, 4College of Applied Medical Sciences, University of Jeddah, Jeddah, Saudi Arabia, 5School of Psychology, College of Engineering, Science and Environment, University of Newcastle, Newcastle, Australia, 6Department of Neurology, John Hunter Hospital, New Lambton Heights, Australia, 7School of Medicine and Public Health, College of Health, Medicine and Wellbeing, University of Newcastle, Newcastle, Australia, 8CSIRO Health and Biosecurity, Brisbane, Australia, 9The Florey Institute of Neuroscience and Mental Health, Victoria, Australia, 10Murdoch Children's Research Institute, Royal Children's Hospital, University of Melbourne, Victoria, Australia, 11Siemens Healthcare GmbH, Erlangen, Germany, 12Department of Neuroradiology, University Clinic Erlangen, Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), Erlangen, Germany
This work provides a new method for fast post-processing of MRI data acquired using the WASAB1 sequence for simultaneous B0 and B1 mapping, used in CEST imaging for field inhomogeneity corrections. We are proposing a new processing method with outstanding acceleration of the parameter estimation procedure, without compromising the stability of the estimation. The stability of the method is demonstrated on phantom data and in vivo 3 Tesla clinical data.
|
||
2718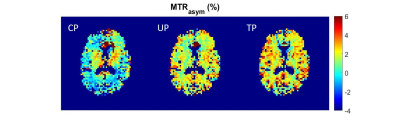 |
82 | CEST MRI at 7T using an optimized parallel transmission saturation scheme
Thaddée Delebarre 1,2, Vincent Gras1,2, Franck Mauconduit1,2, Alexandre Vignaud1,2, Nicolas Boulant1,2, and Luisa Ciobanu1,2
1NeuroSpin, CEA, Saclay, France, 2Paris-Saclay University, Gif-sur-Yvette, France Compared to the traditional circular polarization (CP) transmission mode, parallel transmission (pTx) excitation allows significant gains in contrast and homogeneity for Chemical Exchange Saturation Transfer (CEST) imaging. Using an in-house developed CEST-EPI sequence incorporating a pTx optimized CEST saturation block, we show that both tailored and universal pTx pulses provide significant improvement at 7T for APT-CEST, GluCEST and GlucoCEST imaging and enable saturation powers unattainable with CP. |
||
2719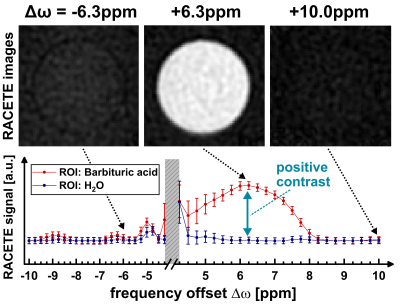 |
83 | Simultaneous imaging of positive and negative chemical exchange contrast: On the implementation of the RACETE-technique on a clinical 3T-system
Verena Schirmer1, Maximilian Gram1,2, Petra Albertova1, Simon Mayer1,3, Martin Blaimer4, Peter Michael Jakob1, and Fabian Tobias Gutjahr1
1Experimental Physics 5, University of Würzburg, Würzburg, Germany, 2Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany, 3Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben, Germany, 4Magnetic Resonance and X-ray Imaging Department Fraunhofer IIS, Fraunhofer Institute for Integrated Circuits IIS, Würzburg, Germany
Contrary to the basic principle of CEST, the RACETE technique allows for the direct detection of positive chemical exchange contrast. This method, which was previously demonstrated only under ultra-high field conditions at 7T-17.5T, has now successfully been implemented on a clinical 3T scanner in initial phantom experiments. Furthermore, we present a novel dual-contrast RACETE-technique for simultaneous imaging of the positive RACETE and the negative CEST contrast.
|
||
2720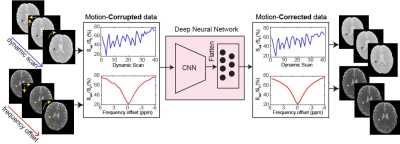 |
84 | Deep learning-based motion correction for Semisolid MT and CEST imaging
Munendra Singh1, Babak Moghadas1, Shanshan Jiang1, Peter van Zijl1, Jinyuan Zhou1, and Hye-Young Heo1
1Johns Hopkins University, Baltimore, MD, United States
Conventional semisolid magnetization transfer contrast (MTC) and chemical exchange saturation transfer (CEST) MRI studies typically employ acquisition of a series of images at multiple RF saturation frequencies. Furthermore, quantitative MTC and CEST imaging techniques based on MR fingerprinting (MRF) require a range of values for multiple RF saturation parameters (e.g. B1, saturation time). These multiple saturation acquisitions lead to a long scan time, which is likely vulnerable to motion during in vivo imaging. Motion correction is hard due to varying image intensity between acquisitions. Herein, we proposed a deep learning-based motion correction technique for conventional Z-spectra and MRF data.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.