Digital Poster
Molecular
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
1988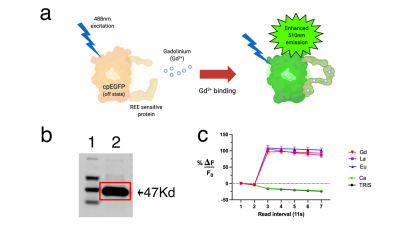 |
83 | Biosynthesis of an Activatable Fluorescent MRI Contrast Agent
Harvey D. Lee1,2, Connor J. Grady1,2, Christiane L. Mallett1,3, Md Nafiujjaman1,2, Nathan M. Good4, N. Cecilia Martinez-Gomez4, Taeho Kim1,2, Erik M. Shapiro1,3, and Assaf A. Gilad1,2,3
1Department of Biomedical Engineering, Michigan State University, East Lansing, MI, United States, 2Division of Synthetic Biology, Institute for Quantitative Health Sciences and Engineering, Michigan State University, East Lansing, MI, United States, 3Department of Radiology, Michigan State University, East Lansing, MI, United States, 4Department of Plant and Microbial Biology, University of California, Berkeley, Berkeley, CA, United States
Sustainable products are becoming an integral part of our daily lives - in this study, we biosynthetically produced an MRI contrast agent from E.coli with an r1 of 6.0 (per-gadolinium, 7T field strength, room temperature) that provides the user with optical feedback through a jump in green fluorescence upon binding free gadolinium in solution. We anticipate this will reduce the amount of resources spent on quality control (in assessing the amount of unchelated gadolinium that could otherwise introduce toxicity), whereas its compatibility with lyophilization is expected to improve storage conditions in terms of longevity, stability, and transportation costs.
|
||
1989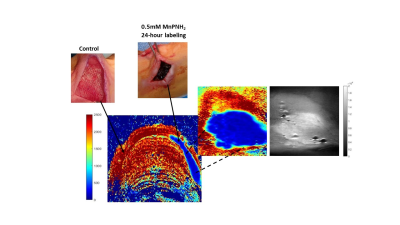 |
84 | 3D Bioprinted MRI-Trackable Regenerative Scaffold for Post-Implantation Monitoring
Sadi Loai1,2, Daniel A. Szulc1,2, and Hai-Ling Margaret Cheng1,2,3
1Institute of Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 2Translational Biology & Engineering Program, Ted Rogers Centre for Heart Research, Toronto, ON, Canada, 3The Edward S. Rogers Sr. Department of Electrical and Computer Engineering, University of Toronto, Toronto, ON, Canada 3D bioprinted scaffolds are one of the most novel and promising tissue regenerative therapeutics currently in development (e.g. to repair damaged cardiac tissue). Validation of correct scaffold placement and retention post-implantation is essential, but it is challenging to visualize scaffolds in-vivo given their similar material properties to native tissue. In this study, a T1-reducing contrast agent, MnPNH2, was utilized to create an MR-trackable, bioprinted scaffold. In-vitro and in-vivo results confirmed the novel scaffold provided an environment conducive to cell growth and offered significant bright contrast for post-implantation scaffold monitoring in rats. |
||
1990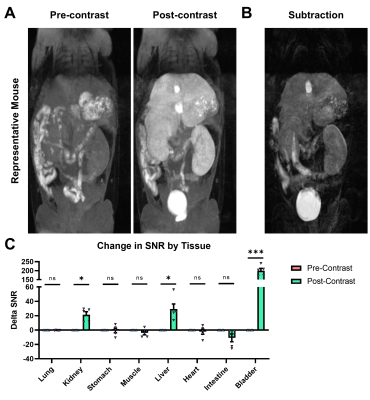 |
85 | A Novel Manganese EOB-Pyclen Diacetate Chelate for Liver-Specific MRI
Ryan Hall1, Jing-Can Qin1, Victoria Laney1, and Nadia Ayat1
1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States
MRI is increasingly utilized for the diagnosis of liver disease and focal liver lesions. While liver-targeted gadolinium-based contrast agents (GBCAs) have high efficacy, they are shadowed by safety concerns regarding tissue retention. We have developed a liver-targeted manganese alternative – Mn-EOB-PC2A – that uses a liver-targeted ethoxybenzyl modified macrocyclic pyclen diacetate platform. Relaxivity measurements for Mn-EOB-PC2A suggest comparable performance to the GBCA alternatives, and in vitro characterization suggests strong uptake in hepatocytes with minimal toxicity. MRI with Mn-EOB-PC2A demonstrated strong liver-specific enhancement at a clinically relevant dose, underscoring the potential for Mn-EOB-PC2A as an alternative to traditional liver-targeted Gd-based platforms.
|
||
| 1991 | 86 | Pentafluorosulfanyl (SF5) is a Superior 19F Magnetic Resonance Reporter Group: Signal Detection of Teriflunomide Derivatives Video Not Available
Ashrith Jacob1, Ludger Starke1, Christian Prinz1, Jason M Millward1, Paula Ramos Delgado1, Ariane Fillmer2, Thoralf Niendorf1, and Sonia Waiczies1
1Berlin Ultrahigh Field Facility, MDC, Berlin, Germany, 2Physikalisch - Technische Bundesanstalt (PTB) Braunschweig and Berlin, Berlin, Germany Modifications to the CF3 side-group of teriflunomide were made to improve its detection by 19F MRI. Pentafluorosulfanyl (SF5) was shown to be a superior alternative to CF3. The SNR efficiency of three 19F MRI methods showed that within a biological environment, SF5-substitutions gave the highest SNR efficiency in combination with an ultrashort echo-time (UTE) MRI method. Chemical modifications did not hamper biological activity but SF5-TF was more effective to inhibit T cell proliferation, indicating better anti-inflammatory activity. This study proposes SF5 as a novel superior 19F MR reporter group for the drug teriflunomide. |
||
1992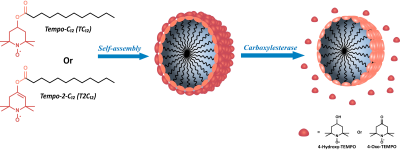 |
87 | Highly sensitive “off/on” EPR probes to monitor enzymatic activity by OMRI Video Permission Withheld
Simonetta Geninatti Crich1, Sabrina Geninatti Elkhanoufi2, Diego Geninatti Alberti2, Eric Thiaudiere3, Rachele Stefania1, Elodie Parzy3, Philippe Mellet3, Philippe Massot3, and Silvio Aime1
1University of Torino, Torino, Italy, 2University of torino, Torino, Italy, 3University of Bordeaux-CNRS, Bordeaux, France
New stable organic radicals probes have been proposed in this study to perform Molecular Imaging by monitoring by Overhauser Magnetic Resonance Imaging (OMRI) the enzymatic activity. The radicals used are Tempo-containing esters forming stable micelles that are practically EPR silent. The hydrolysis of the ester bond catalyzed by Carboxyl esterases generates a narrow and intense EPR signal as a consequence of the release of the nitroxide radical from the micelle. Thus we have prepared a off/on probe responsive to the carboxylesterase activity that can be detected quantitatively in the OMR image.
|
||
1993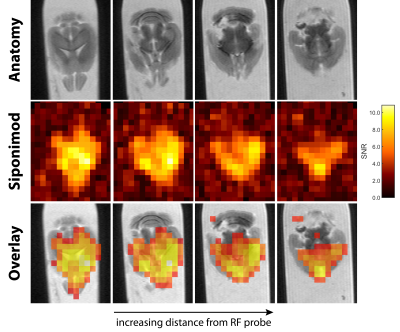 |
88 | Detection of the Multiple Sclerosis Drug Siponimod Using Fluorine-19 Magnetic Resonance Imaging
Ludger Starke1,2, Mariya Aravina1, Jason M. Millward1, Thoralf Niendorf1,3, and Sonia Waiczies1
1Berlin Ultrahigh Field Facility, Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Digital Health Center, Hasso Plattner Institute, Potsdam, Germany, 3Experimental and Clinical Research Center (ECRC), A Joint Cooperation between the Charité Medical Faculty and the Max-Delbrück Center for Molecular Medicine, Berlin, Germany
Siponimod is a fluorinated drug approved for treatment of secondary progressive multiple sclerosis. We detect 19F-MR signal in the liver, brain, kidneys, and thymus, and achieve the first 19F-MR images of a disease modifying drug, both in the liver and brain ex vivo and in a proof-of-concept in vivo experiment. Clear concentration differences between lobes of the liver or regions of the CNS can be observed. We have demonstrated the feasibility of using 19F-MRI to study the distribution of disease modifying drugs and improve our understanding of pharmacokinetics or guide therapeutic decisions.
|
||
1994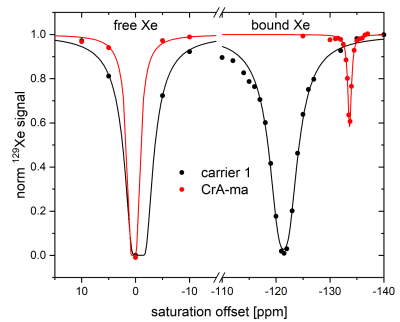 |
89 | HyperCEST Performance of Liposomal Nanocarriers using a CrA-Lipopeptide Building Block Video Permission Withheld
Leif Schröder1 and Jan Oliver Jost1
1Translational Molecular Imaging, Deutsches Krebsforschungszentrum, Heidelberg, Germany MRI reporters for hyperpolarized 129Xe CEST need further improvement in order to sufficiently outcompete the intrinsic loss of hyperpolarization appearing under in vivo conditions. This study opens up a possibility by accelerating the CEST build-up for the well-known Xe host CrA-ma by two orders of magnitude. We designed a liposome decorated with a CrA-lipopeptide. To avoid inefficiency from back exchange, the lipopeptide fraction should be kept relatively low (e.g., 2 mol%). Complete saturation transfer could be easily achieved for maximum possible image contrast in 129Xe MRI scans. This study pinpoints a large flexibility for designing powerful HyperCEST agents. |
||
1995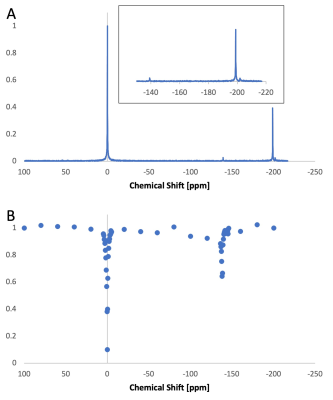 |
90 | Detection of low-boiling point perfluorocarbon nanodroplets using hyperCEST: a path toward a dual-modality dual-phase contrast agent
Christian T McHugh1,2, Phillip G Durham3, Michele Kelley1,2, Nicholas J Bryden1,2, Paul A Dayton2,4, and Rosa T Branca1,2
1Physics & Astronomy, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Pharmacoengineering and Molecular Pharmaceutics, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 4Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States Recently, it was shown that hyperCEST enables detection of microbubbles at clinically relevant ultrasound doses. Nanodroplets filled with low-boiling point perfluorocarbons are precursors of microbubbles. Because the chemical shift of xenon in liquid-phase perfluorocarbon nanodroplets is different than that in gas-phase microbubbles, hyperCEST detection of these nanodroplets could enable MR detection of their phase-change upon ultrasound activation. To this end, here we investigate if perfluorocarbon nanodroplets can be used as hyperCEST agent, first in vitro and then in vivo. |
||
1996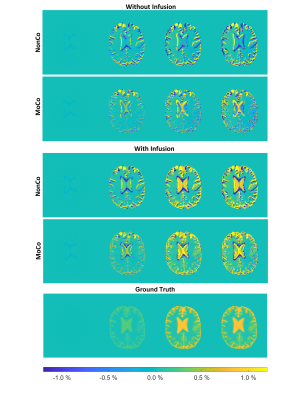 |
91 | GlucoCEST under the influence of head motion at 3 T: A numerical head phantom
Patrick Michael Lehmann1, Mads Andersen2,3, Anina Seidemo1, Xiang Xu4,5, Xu Li5,6, Nirbhay Yadav5,6, Ronnie Wirestam1, Patrick Liebig7, Frederik Testud8, Pia Sundgren3,9,10, Peter C. van Zijl5,6, and Linda Knutsson1,5,6
1Department of Medical Radiation Physics, Lund University, Lund, Sweden, 2Philips Healthcare, Copenhagen, Denmark, 3Lund University Bioimaging Centre, Lund University, Lund, Sweden, 4BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 5Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 6F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 7Siemens Healthcare GmbH, Erlangen, Germany, 8Siemens Healthcare AB, Malmö, Sweden, 9Department of Radiology, Lund University, Lund, Sweden, 10Department of Medical Imaging and physiology, Skåne University hospital, Lund, Sweden D-glucose is proposed as a cheap biodegradable alternative to gadolinium-based contrast agents. By performing glucoCEST imaging during and after administration of glucose, an approach referred to as dynamic glucose-enhanced (DGE) MRI, information about glucose delivery and uptake can be obtained. However, the small DGE signal changes at 3 T can easily be corrupted by motion. Furthermore, standard retrospective motion correction may erroneously alter true DGE signal, which may lead to misinterpretation. We designed a numerical head phantom that can be used for validation of motion correction and providing insight into the corresponding effects in vivo. |
||
1997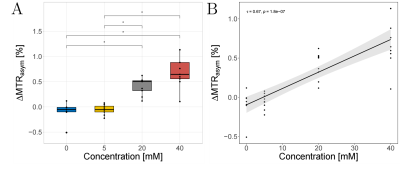 |
92 | Numerical optimization and validation of Lactate CEST (LATEST) imaging at 3T: In-situ and in-vivo feasibility studies
Karl Ludger Radke1, Daniel Benjamin Abrar1, Miriam Frenken1, Lena Marie Wilms1, Matthias Boschheidgen1, Patrick Liebig2,3, Alexandra Ljimani1, Sven Nebelung1,4, Hans-Jörg Wittsack1, and Anja Müller-Lutz1
1University Dusseldorf, Medical Faculty, Department of Diagnostic and Interventional Radiology, Düsseldorf, Germany, 2Cent. of Med. Phys. and Biomed. Eng., Friedrich-Alexander-Univ. Erlangen-Nürnberg, Erlangen, Germany, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Department of Diagnostic and Interventional Radiology, University Hospital Aachen, Aachen, Germany
Quantification of altered lactate concentrations with non-invasive MR imaging is of diagnostic interest in a broad spectrum of diseases and further analysis of athletic activity. In this context, chemical exchange saturation transfer (CEST) is an increasingly validated contrast mechanism in MRI. However, successful application of CEST requires efficient selective saturation of the exchanging lactate protons. In this study, we successfully optimized and investigated the application of intramuscular lactate CEST imaging (LATEST) supported by numerical simulations and were able to apply LATEST for the first time in-vivo at 3.0 T.
|
||
1998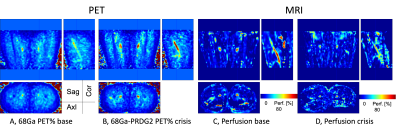 |
93 | Simultaneous molecular 68Ga-PRDG2 and perfusion PET-MR imaging in a patient with sickle cell disease Video Permission Withheld
Chan Hong Moon1, Carolyn Anderson1,2,3,4,5, Sina Tavakoli 5,6, Tiffany Pham 6, Lydia Perkins5, Lynda Little-Ihrig5, Neal Mason1, Xiaoyuan Chen7, Charles Laymon1,2, Mark Gladwin5, and Enrico Novelli 3,5
1Department of Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States, 3Department of Pharmacology and Chemical Biology, University of Pittsburgh, Pittsburgh, PA, United States, 4Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, United States, 5Department of Medicine, University of Pittsburgh, Pittsburgh, PA, United States, 6Gilead Sciences, Foster City, CA, United States, 7National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, MD, United States
Sickle cell disease (SCD) causes vaso-occlusion, ischemia, and end-organ infarction that causes acute pain episodes, also known as vaso-occlusive crises (VOC). Understanding the mechanism underlying VOC is critical to identify patients at risk and for accurate diagnosis while in VOC. PET imaging with the probe 68Ga-PRGD2 that binds integrin αvβ3 identified areas of increased binding of sickle RBC to the endothelium in VOC. Simultaneous PET-MR imaging could provide additional information on tissue changes associated with VOC. Thus, we imaged a patient with SCD with 68Ga-PRGD2 in combination with MRI by using PET-MR 3T scanner.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.