Digital Poster
Pulmonary Imaging I: Methods
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
1395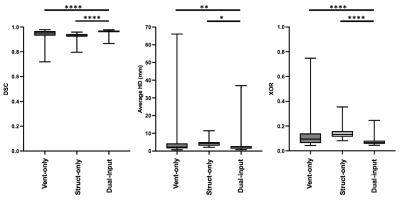 |
19 | A multi-channel deep learning approach for lung cavity estimation from hyperpolarized gas and proton MRI Video Permission Withheld
Joshua R Astley1,2, Alberto M Biancardi2, Helen Marshall2, Paul JC Hughes2, Guilhem J Collier2, Laurie J Smith2, James Eaden2, François-Xavier Blé3, Rod Hughes4, Jim M Wild2,5, and Bilal A Tahir1,2
1Oncology and Metabolism, University of Sheffield, Sheffield, United Kingdom, 2POLARIS, Sheffield, United Kingdom, 3Translational Science and Experimental Medicine, Research and Early Development, Respiratory & Immunology, BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom, 4Clinical Development, Research and Early Development, Respiratory & Immunology, AstraZeneca, Cambridge, United Kingdom, 5Insigneo Institute for in silico medicine, University of Sheffield, Sheffield, United Kingdom
Functional lung imaging biomarkers, such as the ventilated defect percentage, are computed from segmentations derived from spatially co-registered functional hyperpolarized gas MRI and structural 1H-MRI. Although hyperpolarized gas and 1H-MRI can be acquired in the same or similar breaths, the acquired scans are frequently misaligned. Here, we propose a multi-channel deep learning-based approach to generate accurate lung cavity estimations (LCEs). Across all evaluation metrics, the multi-channel approach significantly outperformed single-channel approaches and generated plausible LCEs across a wide range of pulmonary pathologies. In addition, correlation and Bland-Altman analyses of lung volumes demonstrated strong correlation and minimal bias with expert LCEs.
|
||
1396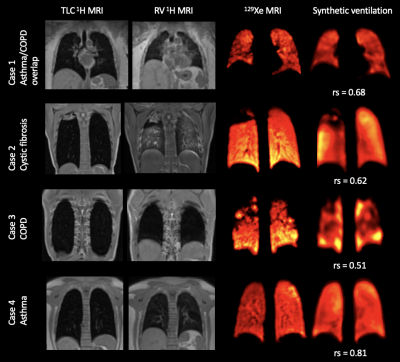 |
20 | Deep learning-based synthesis of hyperpolarized gas MRI ventilation from 3D multi-inflation proton MRI Video Permission Withheld
Joshua R Astley1,2, Alberto M Biancardi2, Helen Marshall2, Malina-Maria Tofan1, Laurie J Smith2, Paul JC Hughes2, Guilhem J Collier2, Matthew Q Hatton1,3, François-Xavier Blé4, Rod Hughes5, Jim M Wild2,6, and Bilal A Tahir1,2
1Oncology and Metabolism, University of Sheffield, Sheffield, United Kingdom, 2POLARIS, Sheffield, United Kingdom, 3Clinical Oncology, Weston Park Cancer Centre, Sheffield, United Kingdom, 4Translational Science and Experimental Medicine, Research and Early Development, Respiratory & Immunology, BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom, 5Clinical Development, Research and Early Development, Respiratory & Immunology, AstraZeneca, Cambridge, United Kingdom, 6Insigneo Institute for in silico medicine, University of Sheffield, Sheffield, United Kingdom
Hyperpolarized gas MRI can visualize and quantify regional lung ventilation with exquisite detail, but clinical uptake is limited to a few centres worldwide. Alternative, non-contrast techniques have been proposed to image ventilation, including 1H-based surrogates of ventilation from multi-inflation 1H-MRI. Recently, deep learning has shown potential for generating synthetic images in multiple modalities within the lung image analysis field. We propose a 3D multi-channel deep learning approach to synthesize hyperpolarized gas MRI and assess its quantitative performance using several common image synthesis metrics across a large, diverse dataset of lung pathologies using 5-fold cross-validation.
|
||
1397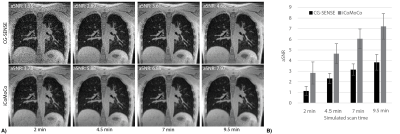 |
21 | Improved 3D stack-of-spiral UTE pulmonary imaging at 0.55T using iterative concomitant field and motion corrected reconstruction (iCoMoCo)
Ahsan Javed1, Rajiv Ramasawmy1, Joel Moss2, Waqas Majeed3, Pan Su3, Thomas Benkert4, Himanshu Bhat3, Ashkan Malayeri5, and Adrienne E. Campbell-Washburn1
1Cardiovascular Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Pulmonary Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 3Siemens Medical Solutions USA Inc, Malvern,, PA, United States, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Department of Health and Human Services, Bethesda, MD, United States
Lung MRI using a high-performance 0.55T system offers reduced susceptibility artifacts, but high resolution imaging is challenged by low proton density, reduced signal-to-noise ratio, and concomitant-fields. 3D spiral acquisitions can be used for improved efficiency and SNR, at the expense of concomitant field related blurring. We present a self-gated, ultra-short echo time, stack-of-spirals acquisition with an efficient inline iterative reconstruction (<15 min) that incorporates both concomitant-field and motion-correction to enable robust high-resolution lung imaging at 0.55T. Our optimized and efficient imaging method is applied to improve image quality in healthy volunteers, patients with lung nodules, and patients with lymphangioleiomyomatosis (LAM).
|
||
1398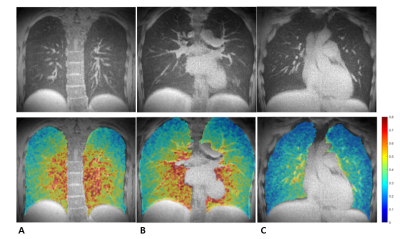 |
22 | 3D Pulmonary Perfusion Mapping Using 3D Ultrashort Echo-time Imaging
Hyeonha Kim1, Seokwon Lee2, Jinil Park3, and Jang-Yeon Park1,2
1Department of Intelligent Precision Healthcare Convergence, Sungkyunkwan University, Suwan, Korea, Republic of, 2Department of Biomedical Engineering, Sungkyunkwan University, Suwan, Korea, Republic of, 3Biomedical Institute for Convergence at SKKU, Sungkyunkwan University, Suwon, Korea, Republic of
Lung MRI is getting more interest as an alternative to CT because of no radiation exposure and expands its role of providing structural information to functional information such as ventilation and perfusion. In terms of 3D functional imaging, 3D ventilation mapping was already proposed, but few studies have been done on 3D perfusion mapping. Here, we propose a 3D pulmonary perfusion map using 3D UTE-MRI with retrospective respiratory and cardiac gating. The proposed method provides high-resolution 3D regional perfusion information of the lungs and will be useful for diagnosing diffusive lung diseases along with ventilation map (e.g., V/Q ratio).
|
||
1399 |
23 | Variation of Proton Lung Signal with Ventilation at 3T
Zachary Peggs1, Jonathan Brooke2, Andrew Cooper1, Charlotte Bolton2, Ian Hall2, Susan Francis1, and Penny Gowland1
1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2The Nottingham Biomedical Research Centre (BRC), Respiratory Medicine Dept, School of Medicine, University of Nottingham, Nottingham, United Kingdom The nature of MR signal variations during healthy ventilation at 3T were explored in 8 healthy participants. Fourier decomposition (FD) techniques were used to remove cardiac signal contributions, and the phase, correlation coefficient and gradient of the parenchyma signal with respect to diaphragm signal were found. This analysis showed that for some voxels, the relationship between parenchyma signal and diaphragm signal is distinctly non-linear. Also, that breathing pattern is an important confounding factor when using the diaphragmatic movement as a surrogate for the change in lung volume, where an additional measure such as a separate 3D acquisition may prove beneficial. |
||
1400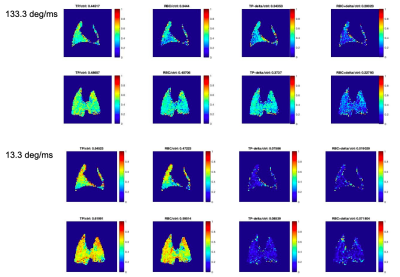 |
24 | Introducing a correction method for XTC imaging to isolate exchange by compartment
Tahmina Achekzai1, Luis Loza1, Stephen Kadlecek1, Kai Ruppert1, Faraz Amzajerdian1, and Rahim Rizi1
1University of Pennsylvania, Philadelphia, PA, United States HP-Xe Xenon Transfer Contrast (XTC) imaging produces exchange maps between gas (GP) and red blood cell (RBC) and tissue plasma (TP). However, it is difficult to separate the effects of exchange between RBC or TP due to unintended depolarization by the saturation pulse. We introduce a correction method to isolate exchange due to either compartment. The correction method measures depolarization due to saturation at a distance Δ, —the separation between the DP resonances—away from the compartment’s resonance frequency. The corrected maps generated here show that greater correction is needed for discrete pulses and higher power pulses. |
||
1401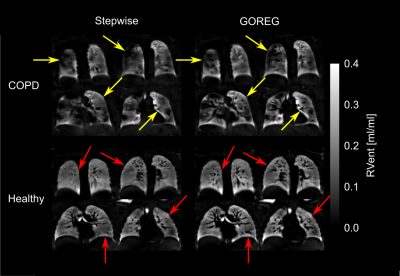 |
25 | Influence of group-oriented registration on functional ventilation parameters derived by 3D phase-resolved functional lung ventilation MRI
Filip Klimeš1,2, Andreas Voskrebenzev1,2, Marcel Gutberlet1,2, Cristian Crisosto1,2, Robert Grimm3, Frank Wacker1,2, and Jens Vogel-Claussen1,2
1Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Member of the German Centre for Lung Research (DZL), Hannover, Germany, 3MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Demonstration of a high repeatability is a mandatory step prior to the usage of new methods in clinical routine. Good to moderate repeatability of 3D phase-resolved functional lung (PREFUL) MRI was shown before. In this study, group-oriented registration (GOREG) was compared to stepwise registration regarding the repeatability of 3D PREFUL ventilation parameters in 30 study participants. Furthermore, image sharpness and structural similarity (SSIM) were assessed. An increased repeatability of dynamic 3D PREFUL ventilation parameters was observed using the GOREG approach. Also, image sharpness and SSIM values were significantly higher for measurements assessed with GOREG registration.
|
||
1402 |
26 | Feasibility of phase resolved functional lung imaging (PREFUL) with ultrashort echo time sequence (PUTE)
Lea Behrendt1,2, Marcel Gutberlet1,2, Andreas Voskrebenzev1,2, Filip Klimeš1,2, Arnd Obert1,2, Agilo Luitger Kern1,2, Frank Wacker1,2, and Jens Vogel-Claussen1,2
1Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany Phase-resolved Functional Lung imaging (PREFUL) allows the contrast-free quantification of pulmonary ventilation and perfusion dynamics in free breathing. Typically, it is used in conjunction with cartesian spoiled gradient echo sequence (SPGRE) with asymmetric echo. Due to the short T2* times in lung tissue, acquiring signal with echo times as low as possible is essential. Therefore, a 2D ultrashort echo time sequence with a TE of 0.07ms was developed and tested in the PREFUL context in one healthy volunteer. A similar visual impression of derived ventilation maps was found with UTE and SPGRE. However, ventilation defects were more pronounced for UTE. |
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.