Online Gather.town Pitches
Cardiovascular Anatomy, Function, Hemodynamics IV
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Booth # | ||||
|---|---|---|---|---|
4859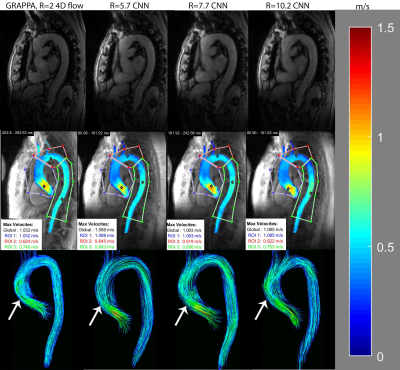 |
1 | AI-based Fully Automated 4D Flow Image Reconstruction and Post-Processing Pipeline in under 10 minutes.
Haben Berhane1, Michael Scott1, Ashitha Pathrose1, Patrick McCarthy2, Chris Malaisrie2, Bradley Allen3, Ryan Avery3, and Michael Markl1
1Biomedical Engineering, Northwestern University, Chicago, IL, United States, 2Cardiac Surgery, Northwestern University, Chicago, IL, United States, 3Radiology, Northwestern University, Evanston, IL, United States
4D flow MRI provides comprehensive assessment of cardiovascular hemodynamics. However, the current clinical usage of standard 4D flow MRI is hindered by long scan times and extensive, time-consuming post-processing such as eddy current corrections, noise masking, and 3D vessel segmentation. We seek to address this by developing a fully automated image reconstruction and post-processing pipeline for highly-accelerated aortic 4D flow MRI (R=5.7-10.2). The fully automated pipeline was shown to provide good-to-excellent agreement in quantitative and hemodynamic measures to conventional 4D flow MRI (GRAPPA, R=2) with manual post-processing. Additionally, the pipeline only requires <10 minutes compared to 20 minutes manually.
|
||
4860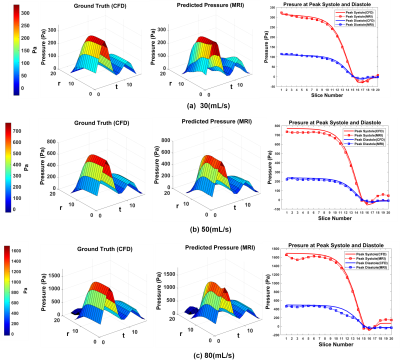 |
2 | Velocity to Pressure Mapping in Stenotic Pulsatile Flows with an Encode-Decoder Deep Network
Ruponti Nath1, Amirkhosro Kazemi1, Marcus Stoddard2, and Amir Amini1
1ECE, University of Louisville, Louisville, KY, United States, 2Cardiovascular Division, Robley Rex VA Medical Centre, Louisville, KY, United States
We propose a novel deep learning based approach to estimate pressure drop inside a stenotic valve from 4D Flow MRI velocities. A neural network architecture learns the relationship of three directional velocities and predicts pressure as output. The network was tested on real 4D flow MRI data of aortic valvular flow both in-vitro and in vivo. Estimated in-vitro pressure drop by proposed method shows (3-5)% relative pressure drop error with corresponding CFD pressure. Estimated In-vivo pressure drop was also compared with doppler Echocardiography and simplified and modified Bernoulli at peak systole timepoints in 10 patients with aortic stenosis.
|
||
4861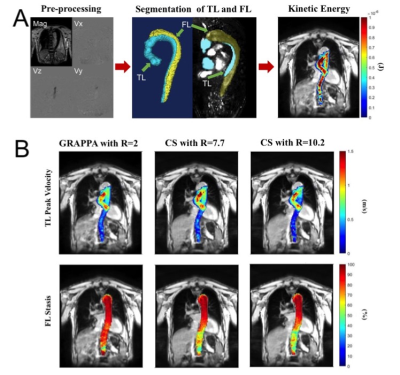 |
3 | Highly accelerated 4D flow MRI using compressed sensing in type B aortic dissection Video Permission Withheld
Ozden Kilinc1, Stanley Chu1, Elizabeth K. Weiss1,2, Justin Baraboo1,2, Anthony Maroun1, Ning Jin3, Kelvin Chow1,4, Xiaoming Bi4, Rachel Davids4, Chris Mehta5, S. Chris Malaisrie5, Andrew Hoel6, Michael Markl1,2, and Bradley D. Allen1
1Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 2Department of Biomedical Engineering, Northwestern University, Chicago, IL, United States, 3Cardiovascular MR R&D, Siemens Medical Solutions USA, Inc., Cleveland, OH, United States, 4Cardiovascular MR R&D, Siemens Medical Solutions USA, Inc., Chicago, IL, United States, 5Division of Cardiac Surgery, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 6Division of Vascular Surgery, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
4D flow MRI is an established quantitative MR imaging technique for evaluating complex blood flow patterns. However, clinical applications of standard 4D flow MRI technique is limited by long scan times associated with multidimensional imaging. Compressed sensing (CS) acceleration can significantly reduce scan time through dramatic data undersampling, but it is not clear how this undersampling impacts assessment of flow patterns in type B aortic dissection (TBAD). In this study, we investigate performance of highly accelerated CS 4D flow MRI at two different acceleration factor levels (R=7.7 and 10.2) to evaluate aortic flow dynamics in volunteers and patients with TBAD.
|
||
4862.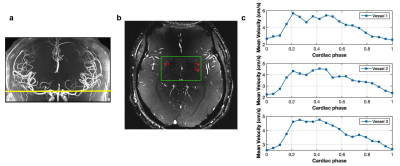 |
4 | Assessment of arterial pulsatility of cerebral perforating arteries using 7T high-resolution dual-VENC phase-contrast MRI
Jianing Tang1, Samantha J Ma2, and Lirong Yan1,3
1USC Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Siemens Medical Solutions USA, Inc., Los Angeles, CA, United States, 3Department of Neurology, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
A dual VENC strategy was applied in 2D high-resolution phase-contrast MRI to improve the sensitivity of arterial pulsatility measurement in cerebral perforating arteries at 7T. Compared to a single VENC of 40cm/s, significant increase in the number of detected perforators was observed using a dual-VENC approach with VENC of 20cm/s and 40cm/s. Reproducibility of arterial pulsatility measurement in perforating arteries was evaluated in test-retest experiments. Good agreement was achieved between test-retest pulsatility measurements.
|
||
4863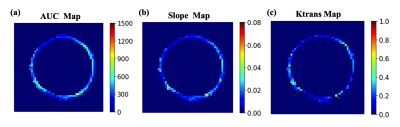 |
5 | Dynamic Contrast Enhanced (DCE) MRI of the Abdominal Aortic Aneurysm (AAA) Wall
Ang Zhou1, Huiming Dong1, Joseph Leach1, Chengcheng Zhu2, David Saloner1, Michael Hope1, and Dimitrios Mitsouras1
1Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Department of Radiology, University of Washington, Seattle, WA, United States
Abdominal Aortic Aneurysms (AAAs) are common in older men. Guidelines recommend repair at an aneurysm diameter greater than 5.5 cm, but some smaller aneurysms experience catastrophic rupture. Aneurysm features beyond diameter have been explored as markers of disease progression. We used DCE MRI to explore the inhomogeneity of contrast kinetics, including Ktrans, in the aneurysm wall and assess if these markers independently predict AAA progression. Significant differences in DCE metrics were identified around the AAA circumference. Area under enhancement-time curve and enhancement slope correlated with growth rate but not diameter, suggesting that DCE may provide diameter-independent information regarding AAA risk.
|
||
4864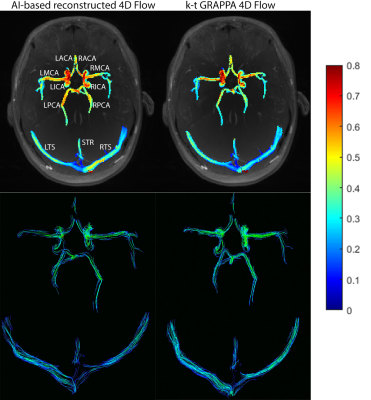 |
6 | AI-based Image Reconstruction of kt-accelerated Intracranial Dual-Venc 4D Flow MRI
Haben Berhane1, Jackson E Moore1, Ann Ragin1, Eric Russell2, Fan Caprio3, Susanne Schnell4, Sameer A Ansari2, and Michael Markl1
1Biomedical Engineering, Northwestern University, Chicago, IL, United States, 2Radiology, Northwestern University, Chicago, IL, United States, 3Neurology, Northwestern University, Chicago, IL, United States, 4Radiology, University of Greifswald, Greifswald, Germany
4D flow MRI provides a comprehensive assessment of hemodynamics through the 3D visualization and quantification of blood flow. However, its current clinical usage is hindered by long scan times. Recent developments have shown deep learning to be highly effective in accelerating image reconstruction, but no study has shown the its effectiveness in reconstructing highly undersampled cerebrovascular 4D flow MRI data. As such, we developed a CNN for the reconstruction of kt-accelerated intracranial dual-venc 4D flow MRI (R=5). We found that the CNN showed excellent SSIM values (0.94[0.93-0.95]) and moderate-to-good net flow and peak velocity agreement with the conventionally reconstructed data.
|
||
4865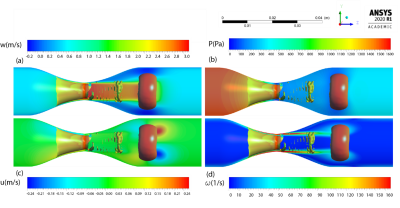 |
7 | Vortex ring reveals a novel non-invasive hemodynamic biomarker associated with the location of maximum pressure drop in arterial stenosis
Amirkhosro Kazemi1,2, Sean Callahan1,2, Ruponti Nath1,2, Marcus Stoddard2,3, and Amir A. Amini1,2
1Electrical and Computer Engineering, University of Louisville, Louisville, KY, United States, 2Robley Rex VA Medical Center, Louisville, KY, United States, 3Cardiovascular Division, University of Louisville, Louisville, KY, United States
We propose vortex ring as a new indicator of the location of maximum pressure drop along a phantom model of arterial stenosis. We investigated details of the flow structure of post stenotic jet with presence of the vortex ring in stenotic phantoms with 87% reduction in area using CFD velocities and 4D flow MRI. The pressure, velocity, and vorticity fields were quantitatively analyzed with the presence of the vortex ring in the spatiotemporal domains. It was found that the location of the vortex ring is associated with the location of the maximum pressure drop, axial velocity, and vorticity magnitude.
|
||
4866 |
8 | Validation of Qp/Qs quantification based on 4D flow MRI: effects of scan acceleration techniques
Takashi Fujiwara1, Erin K Englund1, Mehdi H Moghari1, Brian Fonseca2, Lorna P Browne1, and Alex J Barker1,3
1Department of Radiology, Children's Hospital Colorado, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 2Department of Pediatrics, Children's Hospital Colorado, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 3Department of Bioengineering, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
The ratio of pulmonary to systemic blood flow (Qp/Qs) is a clinically important index to estimate the existence and amount of cardiovascular shunt flow and to determine the need for surgical intervention for congenital heart disease patients. Accelerated 4D flow MRI is capable of measuring Qp/Qs in a single acquisition, but its accuracy and reliability are not well established. We compared conventional 2D phase-contrast MRI and two 4D flow MRI acquisitions with different acceleration techniques to investigate accuracy of Qp/Qs quantification with accelerated 4D flow in adult healthy volunteers (N=10). Consequently, we found no statistical differences in Qp/Qs.
|
||
4867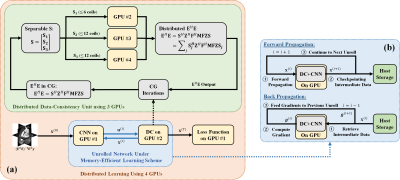 |
9 | Distributed memory-efficient deep learning reconstruction for improved SIMBA whole-heart coronary MRI
Chi Zhang1,2, Ludovica Romanin3,4, Davide Piccini3,4, Steen Moeller2, Matthias Stuber3,5, and Mehmet Akçakaya1,2
1Department of Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 5Center for Biomedical Imaging, Lausanne, Switzerland
SImilarity-driven Multi-dimensional Binning Algorithm (SIMBA) is a recently proposed technique for identifying motion-consistent clusters in free-running whole-heart MRA acquisitions, where the clusters are subsequently reconstructed using conventional approaches. Physics-guided deep learning (PG-DL) reconstruction has gained popularity with its superior performance at higher acceleration rates and may further improve the image quality of free-running whole-heart MRA in conjunction with SIMBA. However, PG-DL is difficult to apply to 3D non-Cartesian acquisitions due to hardware limitations. In this work we enable distributed memory-efficient PG-DL for large-scale SIMBA datasets, showing preliminary results in which PG-DL improves image quality compared to conventional methods.
|
||
4868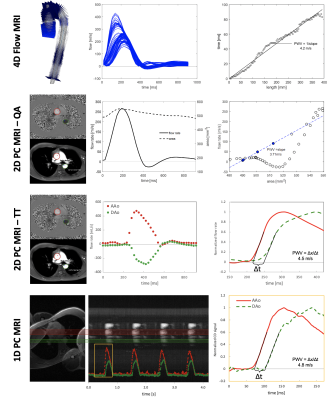 |
10 | Comparison of 4D, 2D, and 1D phase-contrast MRI quantification of aortic pulse wave velocity in healthy adults
Erin K Englund1, Takashi Fujiwara1, Kelly B Jarvis2, Michael Markl2, Daniel Enge3, Jane E.B. Reusch4, and Alex J Barker1
1Radiology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 2Radiology, Northwestern University, Chicago, IL, United States, 3Bioengineering, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 4Endocrinology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
Pulse wave velocity (PWV) is an important measure of cardiovascular health, related to vascular stiffness. Aortic PWV can be evaluated with a variety of MRI methods, however a direct comparison of PWV results quantified from 4D flow, 2D phase contrast (PC), and 1D projection PC MRI has not been performed. Here, PWV derived from 4D, 2D, and 1D-PC MRI acquisitions and associated analysis strategies were compared in the aortic arch. Relative agreement was observed among methods. 2D-PC PWV derived from the flow-area approach had substantial variability, likely due to the limited spatial/temporal resolution, and user-dependence of analysis.
|
||
4869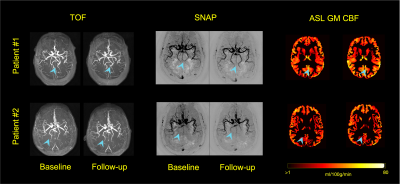 |
11 | Can blood flow and artery patency alterations in medium-to-large arteries predict alteration in cognitive function?
Kaiyu Zhang1, Zhensen Chen2, Li Chen1, Gador Canton1, Duygu Baylam Geleri1, Kristi Pimentel1, Niranjan Balu1, Thomas Hatsukami1, and Chun Yuan1
1Vascular Imaging Lab and BioMolecular Imaging Center, Department of Radiology, University of Washington, Seattle, WA, United States, 2Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China
Association between brain tissue level perfusion and cognitive decline has been previously documented. However, contribution of blood flow and artery patency in medium-to-large arteries and its relation to tissue level perfusion and cognition remain unclear. In this study, 3D-TOF, ASL, and SNAP, an MRA technique sensitive to slow blood flow, were acquired at baseline and 12-months follow-up. A vascular map construction software was used to measure changes in medium-to-large arterial features. Changes in SNAP artery length and branch number during follow-up were significantly associated with changes in cognitive function, while no such association was found with ASL tissue level perfusion.
|
||
4870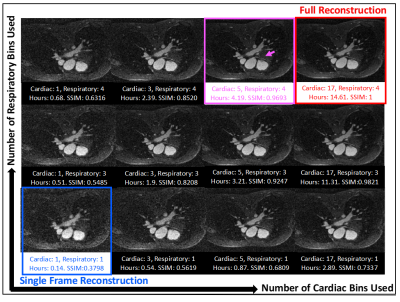 |
12 | Accelerating 5D Whole-Heart MRA Reconstruction Using a Limited Number of Cardiac and Respiratory Frames
Yitong Yang1, Jackson Hair1, Jerome Yerly2, Davide Piccini3, Matthias Stuber4, and John Oshinski1,5
1Biomedical Engineering, Emory University/Georgia Institute of Technology, Atlanta, GA, United States, 2Diagnostic and Interventional Radiology, Lausanne University Hospital, Lausanne, Switzerland, 3Siemens Healthcare, Lausanne, Switzerland, 4Lausanne University Hospital, Lausanne, Switzerland, 5Radiology, Emory University School of Medicine, Atlanta, GA, United States
Under-sampled reconstruction of retrospectively gated 5D free-running acquisition is time-consuming and computationally intensive. In this work, a limited reconstruction method is proposed which provides an efficient way to compute a static cardiac volume from free-breathing, ECG-free 5D free-running CMR acquisitions. Structural similarity index measure is used to compare the limited reconstructions with the fully reconstructed image at the physiologic state of interest. The proposed limited reconstruction method achieves a SSIM of 0.9 using only 22% of the full reconstruction time and an SSIM of 0.95 using 44% of the full reconstruction.
|
||
4871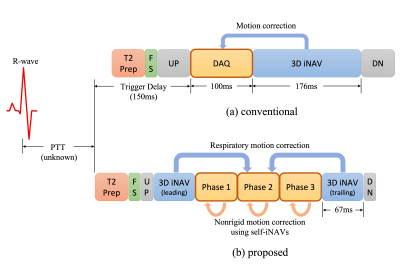 |
13 | Motion-Corrected Multiphase Imaging with Steady-state Free Precession for Free-breathing 3D Coronary Angiography
Kwang Eun Jang1,2, Dwight G. Nishimura2, and Shreyas S. Vasanawala3
1Department of Bioengineering, Stanford University, Stanford, CA, United States, 2Magnetic Resonance Systems Research Lab (MRSRL), Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 3Department of Radiology, Stanford University, Stanford, CA, United States
For free-breathing 3D coronary angiography, we consider a new multiphase acquisition that consists of a series of short imaging blocks to reduce motion blur in the acquisition window. However, this approach poses two challenges: (1) because the alterations of readout gradient waveforms become large due to the shortened imaging window, eddy current artifacts appear in steady-state free precession imaging, and (2) motion among cardiac phases needs to be addressed. In this work, we propose a new ordering scheme to establish smooth transitions between conic interleaves. Phase-to-phase motion is estimated using self image-based navigators and corrected by motion-based reconstruction.
|
||
4872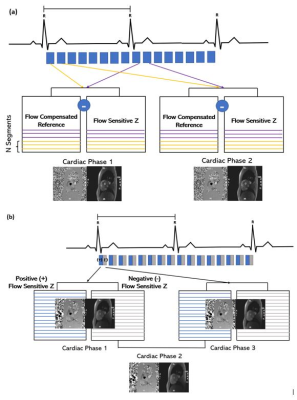 |
14 | Highly Accelerated Free-Breathing Real-Time 2D Flow Imaging using Compressed Sensing and Shared Velocity Encoding
Fei Xiong1, Ning Jin2, Anna Lena Emrich 3, SarahRose Hall3, Jeanie Marie Ruddy3, Jonathan Aldinger4, Gabrielle Young 4, U Joseph Schoepf4, Daniel Giese5, Tilman Emrich 4, and Akos Varga-Szemes4
1Cardiovascular MR R&D, Siemens Medical Solutions USA, Inc, Charleston, SC, United States, 2Cardiovascular MR R&D, Siemens Medical Solutions USA, Inc, Chicago, IL, United States, 3Department of Surgery, Medical University of South Carolina, Charleston, SC, United States, 4Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, SC, United States, 5MR Cardiovascular Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
We aim to demonstrate the feasibility of a highly accelerated, free-breathing RT PC technique empowered by compressed sensing (CS) and shared velocity encoding (SVE) which allows a scan time less than 5s and fast inline image reconstruction.
|
||
4873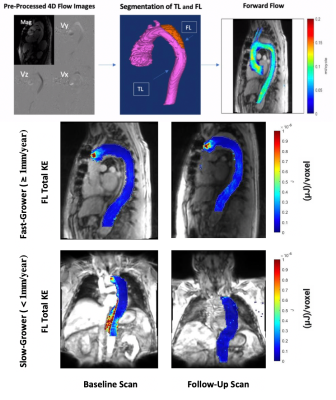 |
15 | Follow-up versus baseline 4D flow-derived in vivo hemodynamic parameters stratify descending aorta dissection patients with enlarging aortas
Stanley Chu1, Ozden Kilinc 2, Elizabeth Weiss2, Kelly Jarvis2, Christopher Mehta 2, Chris Malaisrie 2, Andrew Hoel2, Maurice Pradella 2, Bradley Allen2, and Michael Markl2,3,4
1Radiology, Northwestern University, Chicago, IL, United States, 2Northwestern University, Chicago, IL, United States, 3Siemens Healtineers, Erlangen, Germany, 4Cryolife Inc., Kennesaw, GA, United States
Increasing aorta diameter in type B aortic dissection (TBAD) patients is associated with adverse clinical outcomes. Our study evaluates true and false lumen (TL, FL) hemodynamics (kinetic energy (KE), maximum velocity (MV), forward flow (FF), and reverse flow (RF) in baseline and follow-up thoracic aorta 4D flow (4DF) MRI. Baseline aortic diameter does not correlate with aortic growth rate, while FL-total KE, FF, and RF correlate negatively. At follow-up, larger increases in FL-total KE, total FF, KE ratio and FL-mean MV correlate positively with aortic growth rate. Investigating hemodynamic changes in follow-up 4DF may be useful for evaluating TBAD patients.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.