Oral Session
New Techniques in Data Acquisition & Analysis
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

14:45 |
0101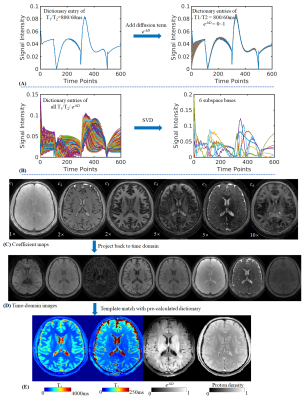 |
3D Diffusion-prepared MRF (3DM) with cardiac gating for rapid high resolution whole-brain T1, T2, proton density and diffusivity mapping
Xiaozhi Cao1,2, Congyu Liao1,2, Zheng Zhong1, Erpeng Dai1, Siddharth Srinivasan Iyer1,3, Ariel J Hannum1,4, Mahmut Yurt1,2, Stefan Skare5, and Kawin Setsompop1,2
1Department of Radiology, Stanford university, Stanford, CA, United States, 2Department of Electrical Engineering, Stanford university, Stanford, CA, United States, 3Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States, 4Department of Bioengineering, Stanford university, Stanford, CA, United States, 5Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden
In this work, a diffusion preparation was implemented in a 3D spiral-projection MRF framework to introduce additional diffusion weighting. Using MRF dictionary with diffusion terms, it enables whole-brain T1, T2, PD and additional diffusivity mapping with 1.25-mm isotropic resolution within 3min. To improve the image quality, cardiac gating and low-resolution navigator were also implemented to mitigate the signal variation caused by motion during diffusion encoding. Subspace reconstruction was used along with LLR regularization to improve the reconstruction conditioning as well as SNR.
|
|
| 14:57 | 0102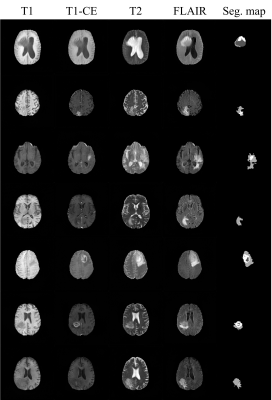 |
Joint Generation of Multi-contrast Magnetic Resonance Images and Segmentation Map Using StyleGAN2-based Generative Network
Geonhui Son1, Taejoon Eo1, Yohan Jun1, Hyungseob Shin1, and Dosik Hwang1
1Electrical and Electronic Engineering, Yonsei university, Seoul, Korea, Republic of
Training deep neural networks for medical imaging commonly requires large image datasets and paired label datasets. However, in the medical imaging research field, labeling costs are very expensive. To overcome this issue, we propose a data generation method which is StyleGAN2-based architecture that jointly generates multi-contrast magnetic resonance (MR) images and segmentation maps. The effectiveness of our generation model is validated in terms of segmentation performance for tumors. We demonstrate that the segmentation model only trained with the fake data generated from our method achieves comparable performance to that trained with real data.
|
|
15:09 |
0103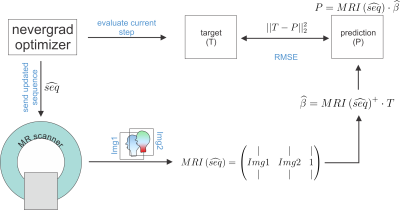 |
MR-double-zero - Can a machine discover new MRI contrasts, such as metabolite concentration?
Sebastian Mueller1,2, Felix Glang1, Kai Herz1,2, Klaus Scheffler1,2, and Moritz Zaiss1,3
1High-field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Department of Biomedical Magnetic Resonance, Eberhard Karls University Tübingen, Tuebingen, Germany, 3Department of Neuroradiology, University Hospital Erlangen, Erlangen, Germany
Discovery of MR contrast and/or conventional sequence parameter optimization usually requires a theoretical model to describe MR physics. Here we investigate if novel contrasts can be found by directly running numerical optimization on a real MRI scanner instead of a simulation. To this end, a derivative-free optimization algorithm is set up to repeatedly update and execute a parametrized sequence on the scanner and map the acquired signals to a given target contrast. As proof-of-principle, we show that this enables creatine concentration mapping by learning a CEST-prepared sequence, which is found solely based on known target concentrations in a phantom.
|
|
15:21 |
0104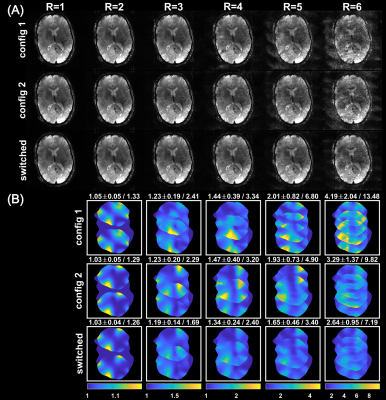 |
Advances in accelerated imaging at 9.4T with electronically modulated time-varying receive sensitivities
Felix Glang1, Anton Nikulin1,2, Jonas Bause1, Rahel Heule1,2, Theodor Steffen1, Nikolai Avdievich1, and Klaus Scheffler1,2
1Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 2Department of Biomedical Magnetic Resonance, Eberhard Karls University Tübingen, Tübingen, Germany
Previously, we have shown in simulations that electronically modulated time-varying receive sensitivities can improve parallel imaging reconstruction when fast modulations are applied during acquisition of k-space lines. Here, we demonstrate this concept experimentally with a prototype 8-channel reconfigurable receive coil, for which B1- modulation is achieved by fast switching PIN diodes in the receive loops. With this setup, MR measurements were performed in both phantom and human subject. Lower reconstruction errors and g-factors (~25% improvement for R=4) were observed for the case of rapidly switched sensitivities compared to conventional reconstruction with static sensitivities.
|
|
| 15:33 | 0105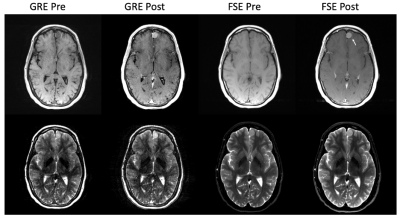 |
Simultaneous 3D T1- and T2-weighted imaging using RF phase-modulated GRE with retrospective contrast adjustment Video Not Available
Daiki Tamada1, Aaron S. Field1,2, and Scott B. Reeder1,2,3,4,5
1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States, 5Medicine, University of Wisconsin-Madison, Madison, WI, United States
A novel method to obtain simultaneous 3D T1- and T2-weighted imaging with retrospective contrast adjustment was developed. Acquisitions are performed using a 3D RF phase-modulated GRE acquisition with a small RF phase increment. After phase correction, real and imaginary components are separated into T2- and T1-weighted images, respectively. Image contrast can also be modified by modulating additional phase into the signals retrospectively. Simulation and in vivo examples are shown. The results suggested the proposed method successfully provides arbitrary image contrasts with clinically acceptable quality and short acquisition time.
|
|
| 15:45 | 0106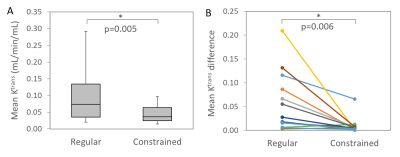 |
Improved Quantification of Ktrans with Cardiac Output Based Correction of Arterial Input Function
Artem Mikheev1, Louisa Bokacheva1, Jeff Lei Zhang2, Hersh Chandarana1, Sungheon Gene Kim3, and Henry Rusinek1
1Department of Radiology, New York University School of Medicine, New York, NY, United States, 2School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 3Department of Radiology, Weill Cornell Medical College, New York, NY, United States
The arterial signal in DCE MRI often suffers from the inflow effect, which may cause large errors in the arterial concentration and compartmental model analysis. We implemented a constrained signal-to-concentration conversion using the subject’s cardiac output based on the Stewart-Hamilton principle, which limits the area under the arterial first pass peak. The constrained conversion significantly reduced the variation of the arterial concentration sampled at three levels along the abdominal aorta. The constrained arterial input function resulted in a significantly lower variability of the Tofts model Ktrans in psoas muscle compared to the uncorrected input function.
|
|
| 15:57 | 0107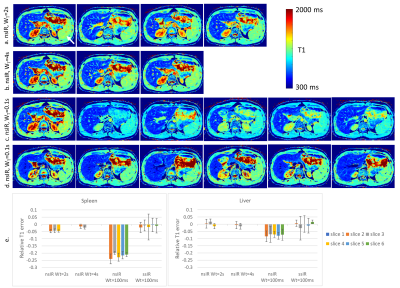 |
Inversion Recovery Look-Locker T1-Mapping for Abdominal Imaging: How Many Slices Can One Fit in a Single Breath-Hold?
Ute Goerke1, Eze Ahanonu2, Mahesh Keerthivasan3, Ali Bilgin2,4,5, Vibhas Deshpande6, and Maria Altbach4,5
1Siemens Healthcare USA, Tucson, AZ, United States, 2Department of Electrical Engineering, University of Arizona, Tucson, AZ, United States, 3Siemens Healthineers USA, New York, NY, United States, 4Department of Biomedical Engineering, University of Arizona, Tucson, AZ, United States, 5Department of Medical Imaging, University of Arizona, Tucson, AZ, United States, 6Siemens Healthineers USA, Austin, TX, United States
In the inversion-recovery Look-Locker T1-mapping, the use of slice-selective inversion recovery pulse is explored to map the long T1-values of the spleen with as many slices as possible. The optimal slice gap in combination with interleaving the slices was determined. Artifact-free T1-maps of the spleen were obtained after parameter optimization with double the number of slices than using the standard approach in a single breath-hold. This new approach is an important step towards achieving complete coverage of abdominal organs within a single breath-hold.
|
|
| 16:09 | 0108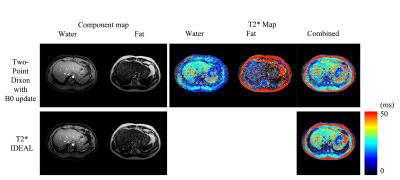 |
T2* mapping for Fat and Water images using Two-Point Dixon method with intrinsic B0 updating Video Not Available
Tzu-Cheng Chao1, Dinghui Wang1, and James G. Pipe1
1Department of Radiology, Mayo Clinic, Rochester, MN, United States
Accurate fat fraction and T2* evaluation offers useful diagnostic information. A data driven B0-update algorithm in conjunction with the Two-Point Dixon method is illustrated in this work, offering efficient computation to improve B0 accuracy to separate fat and water in the multiple TE acquisition. The individual fat and water signals are then used for T2* mapping. The reconstructed parametric maps have comparable quality to those of the existing methods. The results also show that the proposed method requires shorter computation time and is more stable in the presence of an inaccurate reference field map.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.