Oral Session
New Deep/Machine Learning Techniques
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 14:45 | 0681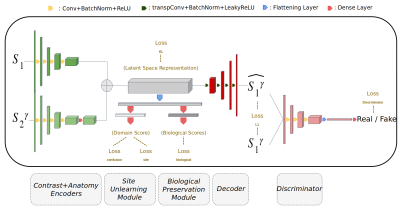 |
ImUnity: a generalizable VAE-GAN solution for multicenter MR image harmonization
Stenzel CACKOWSKI1, Emmanuel Luc Barbier1, Michel Dojat1, and Thomas Christen1
1Grenoble Institut of Neurosciences, Grenoble, France
ImUnity is an original deep-learning model designed for efficient and flexible MRI harmonization. A VAE-GAN network is coupled with a confusion and a biological preservation module. It ‘corrects’ MR images that can be used for various multi-center studies. Using 3 open source databases, we show that ImUnity: outperforms state-of-the-art methods in terms of quality of images generated; removes sites/scanner bias while improving patients classification; harmonizes data coming from new sites/scanners and allows the selection of multiple MR reconstructed images according to the desired applications. Tested on T1w images, ImUnity could be generalized to other types of medical images.
|
|
| 14:57 | 0682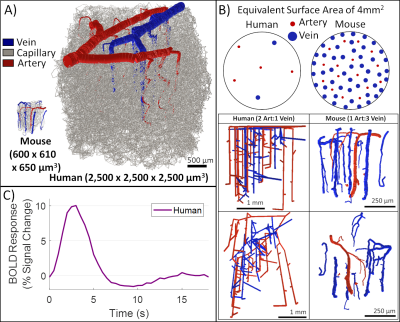 |
Simulated fMRI responses using human Vascular Anatomical Network models with varying architecture and dynamics
Grant Hartung1,2, Joerg Pfannmoeller1,2, Avery J. L. Berman1,2, and Jonathan R. Polimeni1,2,3
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States We utilized a synthesis algorithm to generate a vascular anatomical network model for the human cortex to act as a basis for biophysical simulations of the fMRI response. We identified key features of the vascular topology needed to generate realistic BOLD responses with this model. We used this model to show how parametric variations in vascular architecture affect the BOLD responses across cortical depths. Finally we investigated approaches to translate arteriolar dilation patterns recorded from the mouse cortex to our human model and found that they may not be capable of capturing the distinct hemodynamic response observed in human fMRI data. |
|
| 15:09 | 0683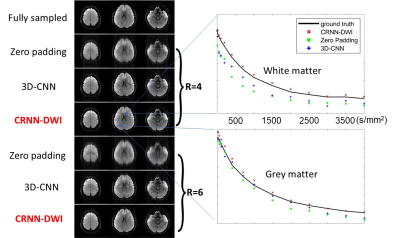 |
Accelerating High b-Value DWI Acquisition Using a Convolutional Recurrent Neural Network
Zheng Zhong1, Kanghyun Ryu1, Jae Eun Song1, Janhavi Singhal2, Guangyu Dan3, Kaibao Sun3, Shreyas S. Vasanawala1, and Xiaohong Joe Zhou3,4
1Radiology, Stanford University, Stanford, CA, United States, 2Homestead High School, Cupertino, CA, United States, 3Center for MR Research, University of Illinois at Chicago, Chicago, IL, United States, 4Radiology, Neurosurgery and Bioengineering, University of Illinois at Chicago, Chicago, IL, United States
DWI can probe tissue microstructures in many disease processes over a broad range of b-values. In the scenario where severe geometric distortion presents, non-single-shot EPI techniques can be used, but introduce other issues such as lengthened acquisition times, which often requires undersampling in kspace. Deep learning has been demonstrated to achieve many-fold undersampling especially when highly redundant information is present. In this study, we have applied a novel convolutional recurrent neural network (CRNN) to reconstruct highly undersampled (up to six-fold) multi-b-value, multi-direction DWI dataset by exploiting the information redundancy in the multiple b-values and diffusion gradient directions.
|
|
| 15:21 | 0684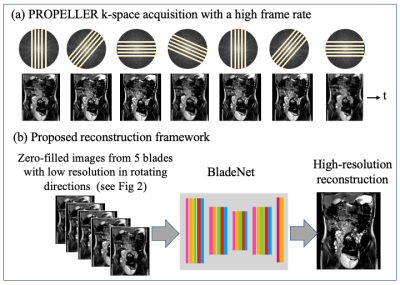 |
BladeNet: Rapid PROPELLER Acquisition and Reconstruction for High spatio-temporal Resolution Abdominal MRI Video Not Available
Efrat Shimron1, Alfredo De Goyeneche1, Ke Wang1, Alma Halgren1, Ali B. Syed2, Shreyas Vasanawala2, and Michael Lustig1
1UC Berkeley, Berkeley, CA, United States, 2Stanford, Stanford, CA, United States
To improve bowel wall imaging in abdominal pediatric MRI scans, we propose a multi-phase single-shot fast spin echo (SSFSE) PROPELLER acquisition with novel deep learning reconstruction. This acquisition offers shorter scan time and thus higher temporal resolution, PROPELLER built-in motion correction, and alias-free images; these however exhibit spatial blurring. Our approach leverages the blurring-axis temporal rotation and data redundancy; we train a network to recover high-frequency spatial details from consecutive frames. Retrospective simulations with data from balanced SSFP scans show that this approach yields reconstructions with high spatio-temporal resolution and motion-correction, which are essential for pediatric abdominal imaging.
|
|
| 15:33 | 0685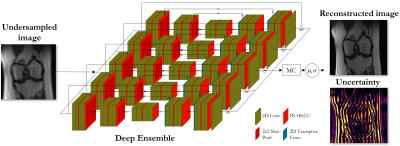 |
Uncertainty estimation via ensembling for deep learning-based MR image reconstruction
Tobias Hepp1,2, Sergios Gatidis1,2, Kerstin Hammernik3,4, and Thomas Küstner1
1Medical Image and Data Analysis (MIDAS.lab), Department of Diagnostic and Interventional Radiology, University Hospital of Tuebingen, Tübingen, Germany, 2Max Planck Institute for Intelligent Systems, Tübingen, Germany, 3Lab for AI in Medicine, Technical University of Munich, Munich, Germany, 4Department of Computing, Imperial College London, London, United Kingdom
Deep learning-based MR image reconstruction from undersampled data bears the risk of inducing reconstruction errors like in-painting of non-anatomical structures, or missing pathologies. These errors may be obscured by the deep learning process and thus remain undiscovered. Furthermore, most methods are task-specialized and not well calibrated to domain shifts. Thus, integrated uncertainty prediction would be desirable. We propose a deep ensembling strategy that allows us to assess potential algorithm failures and better adapt to changing scenarios. The proposed approach can be paired with any DL reconstruction, enabling investigations of their predictive uncertainties on a voxel-level.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.