Oral Session
Alzheimer's Disease & Other Dementias
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 14:30 | 0458 |
White matter changes in myelin, iron and R2* in Alzheimer's disease
Lindsay Munroe1, Azhaar Ashraf1, Antigoni Ekonomou1, Harold Parkes1, Claire Troakes1, and Po-Wah So1
1Neuro-imaging, King's College London, London, United Kingdom
Brain iron dyshomeostasis/iron overload has been observed in Alzheimer's disease (AD). Being sensitive to the presence of iron, magnetic resonance imaging (MRI), is often used to study the role of iron in disease. In this study, we determined group differences in iron, R2* and myelin, acquired from the same ex-vivo mid-temporal gyri samples from AD and cognitively normal (CN) subjects. We observed decreased myelin and iron in white matter AD compared to CN. These white matter changes are likely due to a loss of iron-rich oligodendrocytes and demyelination during AD pathogenesis.
|
|
| 14:42 | 0459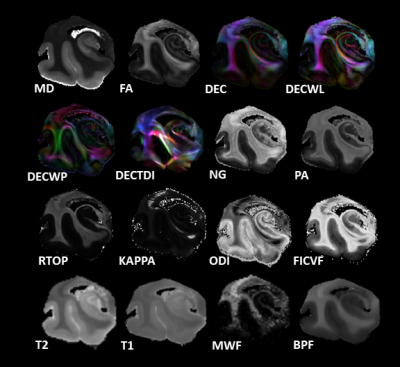 |
Microstructural MR Markers of Alzheimer’s Disease Pathology in Post-Mortem Human Temporal Lobe
Courtney J Comrie1, Laurel A Dieckhaus1, Tom G Beach2, Geidy E Serrano2, and Elizabeth B Hutchinson1
1Biomedical Engineering, University of Arizona, Tucson, AZ, United States, 2Brain and Body Donation Program, Banner Sun Health Research Institute, Sun City, AZ, United States
Alzheimer’s is an irreversible degenerative brain disease. Current clinical MRI is capable of reporting severe brain atrophy, but fails to recognize earlier biomarkers associated with more subtle microstructural changes. Microstructural MRI techniques such as DTI, MAP-MRI, NODDI, MWF, and BPF are promising to address this challenge and may sensitively detect and distinguish tissue degeneration, tauopathies, and beta amyloid plaques. The capability of these techniques was investigated in post-mortem human temporal lobe specimens at high resolution and high image quality. Prominent findings seen were distinct differences between relaxivity and diffusivity metrics, and striking differences between DTI and MAP-MRI anisotropy metrics.
|
|
| 14:54 | 0460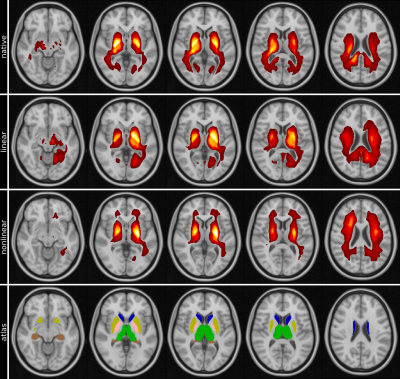 |
Explainable MRI: Revealing the mechanisms underlying deep learning brain disease classification
Christian Tinauer1, Lukas Pirpamer1, Marc Masana2, Stefan Heber1, Anna Damulina1, Maximilian Sackl1, Martin Soellradl3, Reinhold Schmidt1, Stefan Ropele1, and Christian Langkammer1
1Department of Neurology, Medical University of Graz, Graz, Austria, 2Institute of Computer Graphics and Vision, Graz University of Technology, Graz, Austria, 3Institute of Medical Engineering, Graz University of Technology, Graz, Austria
Increased iron deposition in the basal ganglia is a frequent finding in patients with AD. Using R2* maps we separated Alzheimer's patients (n=115) from healthy controls (n=169) by using a deep neural network and systematically investigated the influence of the learned features using an attached relevance map generator. The highest relevances were found in and adjacent to the basal ganglia, which is in line with established histological findings and additionally confirmed by the conventional ROI-based analysis. This study demonstrates the validity of heat mapping as a means to identify novel areas of pathological tissue changes.
|
|
15:06 |
0461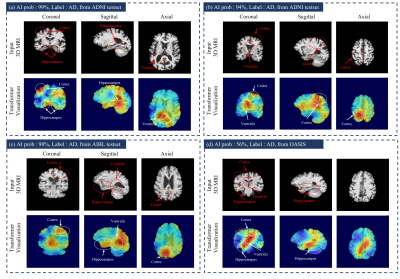 |
Transformer-based Alzheimer’s disease analyzer for multi-institutional 3D MRI images
Jinseong Jang1 and Dosik Hwang1
1Yonsei University, Seoul, Korea, Republic of
We propose Transformer-based Alzheimer’s disease (AD) analyzer for 3D MRI. The proposed network can analyze 3D MRI images efficiently combining 3D CNN, and Transformer. It is possible to efficiently extract locality information for AD-related abnormalities in local brain based on CNN networks with inductive bias. Also, the transformer network is also used to obtain attention relationship among 3D representation features after CNN. Our proposed method was compared to various networks including 3D CNN and transformer with an area under curve and accuracy for AD classification in multi-institutional datasets. Also, the transformer interpretability technique-based activation map can visualize AD-related abnormality region.
|
|
| 15:18 | 0462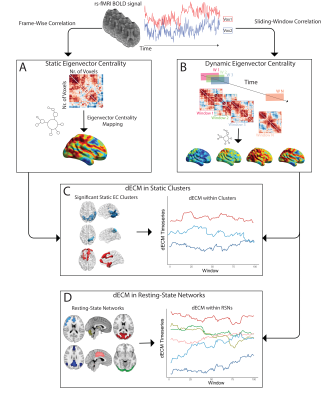 |
Functional eigenvector centrality dynamics are related to amyloid deposition in preclinical Alzheimer’s Disease
Luigi Lorenzini1, Ingala Silvia1, Lyduine E Collij1, Betty Tijms2, Henk JMM Mutsaerts 1,3, Viktor Wottschel 1, Sven Haller4,5, Kaj Blennow 6,7, Giovanni Frisoni8,9, Gael Chételat 10, Pierre Payoux 11,12, Pablo Lage-Martinez 13, Adam Waldman 14,15, Joanna Wardlaw 14,16, Craig Ritchie17, Juan Domingo Gispert 18,19,20,21, Pieter Jelle Visser 2,22,23, Philip Scheltens 2, Barkhof Frederik1, and Alle Meije Wink 1
1Dept. of Radiology and Nuclear Medicine, Amsterdam University Medical Centre, Vrije Universiteit, Amsterdam Neuroscience, Amsterdam, The Netherlands, Amsterdam, Netherlands, 2Department of Neurology, Alzheimer Center Amsterdam, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, the Netherlands, Amsterdam, Netherlands, 3Ghent Institute for Functional and Metabolic Imaging (GIfMI), Ghent University, Ghent, Belgium, Ghent, Belgium, 4CIRD Centre d’Imagerie Rive Droite, Geneva, Switzerland, Geneva, Switzerland, 5Department of Surgical Sciences, Radiology, Uppsala University, Uppsala, Sweden, Uppsala, Sweden, 6Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, the Sahlgrenska Academy at the University of Gothenburg, Sweden, Gothenburg, Sweden, 7Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden, Mölndal, Sweden, 8aboratory Alzheimer’s Neuroimaging & Epidemiology, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy, Brescia, Italy, 9University Hospitals and University of Geneva, Geneva, Switzerland, Geneva, Switzerland, 10Université de Normandie, Unicaen, Inserm, U1237, PhIND "Physiopathology and Imaging of Neurological Disorders", institut Blood-and-Brain @ Caen-Normandie, Cyceron, 14000 Caen, France, Caen, France, 11Department of Nuclear Medicine, Toulouse CHU, Purpan University Hospital, Toulouse, France, Toulouse, France, 12Toulouse NeuroImaging Center, University of Toulouse, INSERM, UPS, Toulouse, France, Toulouse, France, 13Centro de Investigación y Terapias Avanzadas, Neurología, CITA‐Alzheimer Foundation, San Sebastián, Spain, San Sebastián, Spain, 14Centre for Clinical Brain Sciences, The University of Edinburgh, Edinburgh, UK, Edinburgh, Scotland, 15Department of Medicine, Imperial College London, London, UK, London, United Kingdom, 16UK Dementia Research Institute at Edinburgh, University of Edinburgh, UK, Edinburgh, Scotland, 17Centre for Dementia Prevention, The University of Edinburgh, Scotland, UK, Edinburgh, Scotland, 18Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain, Barcelona, Spain, 19CIBER Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Madrid, Spain, Madrid, Spain, 20IMIM (Hospital del Mar Medical Research Institute), Barcelona Spain, Barcelona, Spain, 21Universitat Pompeu Fabra, Barcelona, Spain, Barcelona, Spain, 22Alzheimer Center Limburg, Department of Psychiatry & Neuropsychology, School of Mental Health and Neuroscience, Maastricht University, Maastricht, The Netherlands, Maastricht, Netherlands, 23Division of Neurogeriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden, Stockholm, Sweden
Recent evidence suggests that amyloid deposition in the brain follows — and subsequently affects — temporal variability of neuronal activity and functional connectivity. Temporal variability in functional brain network properties connote changes in participation of functional hubs in different subnetworks. We confirm previous findings of alterations of functional eigenvector centrality (EC) in relation to amyloid, and demonstrate that those regions show altered dynamic EC profiles with lower variability over time, hence lower network integration.
|
|
15:30 |
0463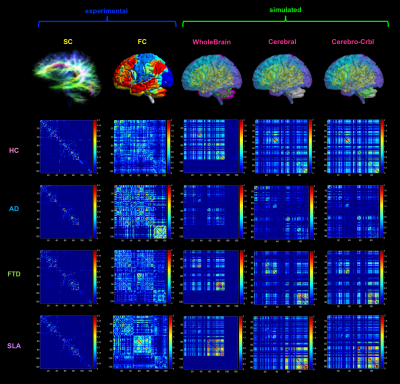 |
Subject-specific features of excitation/inhibition profiles in neurodegenerative diseases
Anita Monteverdi1,2, Fulvia Palesi2, Claudia AM Gandini Wheeler-Kingshott1,2,3, and Egidio D'Angelo1,2
1Brain Connectivity Center, IRCCS Mondino Foundation, Pavia, Italy, 2Brain and Behavioural Sciences, University of Pavia, Pavia, Italy, 3NMR Research Unit, Queen Square Multiple Sclerosis Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, UCL, London, United Kingdom
The Virtual Brain (TVB) is a novel modeling platform to simulate whole-brain dynamics starting from individual structural and functional connectivity. In this work, we performed TVB simulations in neurodegenerative diseases (i.e. Alzheimer’s disease, Frontotemporal Dementia, and Amyotrophic Lateral Sclerosis) providing a unique description of the excitatory/inhibitory balance at single-subject level. TVB-derived biophysical parameters were different among clinical phenotypes, presented an association with neuropsychological domains and improved discriminative power between clinical conditions. Overall, this work suggests a novel TVB-based approach for future personalized diagnosis and therapy in neurodegenerative diseases, providing a subject-specific description of excitation/inhibition profiles.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.