Oral Session
Neurofluids: From Macro to Micro
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

16:45 |
0320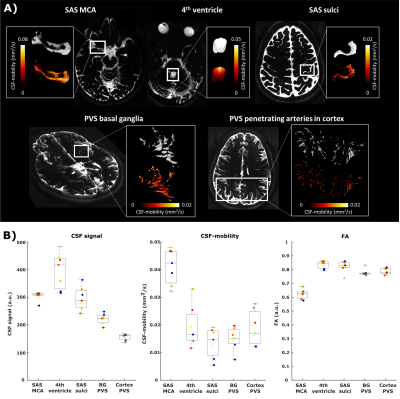 |
Effects of the cardiac and respiratory cycles on CSF-mobility in human subarachnoid and perivascular spaces
Lydiane Hirschler1, Bobby A Runderkamp2, Susanne J van Veluw1,3, Matthan WA Caan4, and Matthias JP van Osch1
1Radiology, Leiden University Medical Center, Leiden, Netherlands, 2Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 3Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 4Biomedical Engineering & Physics, Amsterdam University Medical Center, Amsterdam, Netherlands Although research into brain waste clearance has recently gained increasing attention, the driving force(s) as well as the physiological processes involved still remain strongly debated. Here, we assess the influence of the cardiac cycle and of respiration on the mobility of cerebrospinal fluid (CSF), thought to be an important waste carrier. We found that the cardiac cycle was inducing higher CSF-mobility changes than respiration. |
|
| 16:57 | 0321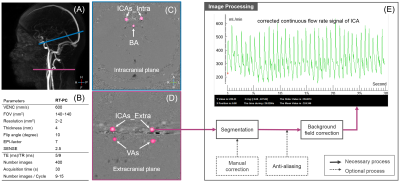 |
Real-Time Phase Contrast MRI to quantify Cerebral arterial flow variations during normal breathing
Liu Pan1,2, Sidy Fall1, Serge Metanbou3, and Olivier Baledent1,2
1CHIMERE UR 7516, Jules Verne University, Amiens, France, 2Medical Image Processing Department, Jules Verne University Hospital, Amiens, France, 3Department of Radiology, Jules Verne University Hospital, Amiens, France Real-time phase contrast MRI has been applied to investigate cerebral arterial blood flow (CABF) during normal breathing of healthy volunteers. We developed a novel time-domain analysis method to quantify the effect of normal breathing on several parameters of CABF. We found the existence of a delay between the recorded respiratory signal from the belt sensor and the breathing frequency component presents in the reconstructed arterial blood flows. During the expiratory, the mean flow rate of CABF increased by 4.4±1.7%, stroke volume of CABF increased by 9.8±3.1% and the duration of the cardiac period of CABF increased by 8.1±3%. |
|
| 17:09 | 0322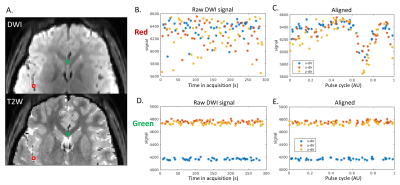 |
Assessment of Pulsatile Cerebrospinal Fluid Dynamics of the Human Brain using CArdiac-cycle Resolved Diffusion-weighted Imaging (CARDI)
Qiuting Wen1, Yunjie Tong2, Xiaopeng Zhou3, Chang Yueh Ho1, and Yu-Chien Wu1
1Indiana University, School of Medicine, Indianapolis, IN, United States, 2Weldon School of Biomedical Engineering Department, Purdue University, West Lafayette, IN, United States, 3School of Health Sciences, Purdue University, West Lafayette, IN, United States
We introduce an MRI technique named CARDI, for in-vivo assessment of pulsatile fluid dynamics in the surface paravascular spaces of the pial arteries. A pair of pulsed gradient spin echo was used to detect the slow flow of CSF while suppressing the fast flow of adjacent arterial blood. Waveform of CSF over a cardiac cycle is revealed. The shape of the CSF waveforms closely resembles the pressure waveforms of the artery wall, suggesting that CSF dynamics is tightly related to artery wall mechanics. The comparison of CSF waveforms in an aging group revealed a significant age-dependency of the CSF waveforms.
|
|
| 17:21 | 0323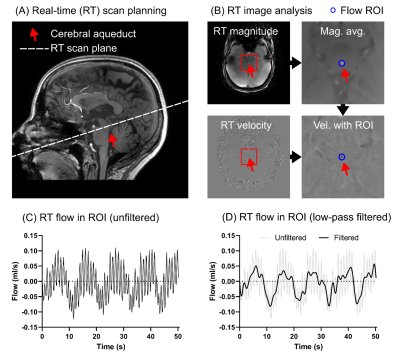 |
High spatial resolution real-time imaging of respiratory effects on cerebrospinal fluid flow at 7T
Johannes Töger1, Mads Andersen2,3, Olle Haglund4, Tekla Kylkilahti5,6, Iben Lundgaard5,6, and Karin Markenroth Bloch3
1Department of Clinical Sciences, Diagnostic Radiology, Skane University Hospital Lund, Lund University, Lund, Sweden, 2Philips Healthcare, Copenhagen, Denmark, 3Lund University Bioimaging Center, Lund University, Lund, Sweden, 4Department of Medical Radiation Physics, Lund University, Lund, Sweden, 5Department of Experimental Medical Science, Lund University, Lund, Sweden, 6Wallenberg Centre for Molecular Medicine, Lund University, Lund, Sweden
Methods to quantify CSF flow dynamics are important to study the physiology of intracranial pressure variations. Respiration influences the CSF flow, making real-time acquisitions necessary. This work investigates a high-resolution radial real-time flow sequence with golden angle acquisition and compressed sensing reconstruction at 7T for flow quantification of the CSF flow in the aqueduct. Phantom data shows that the proposed method accurately quantifies the slower aqueduct CSF flow oscillations caused by respiration, but underestimates faster cardiac oscillations. In vivo data shows good repeatability and an association between respiratory condition and net CSF flow.
|
|
| 17:33 | 0324 |
Contrast Agent Transport in Mouse Glymphatic System Observed by Dynamic Contrast-Enhanced MRI
Yuran Zhu1, Yuning Gu1, Kihwan Kim1, Chaitanya Kolluru1, Huiyun Gao2, Yunmei Wang2,3, David L. Wilson1,4,5, Chris A. Flask1,4,6,7,8, and Xin Yu1,4,9
1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Cardiovascular Research Institute, Case Western Reserve University, Cleveland, OH, United States, 3Department of Medicine, Case Western Reserve University, Cleveland, OH, United States, 4Department of Radiology, Case Western Reserve University, Cleveland, OH, United States, 5Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, OH, United States, 6Department of Pediatrics, Case Western Reserve University, Cleveland, OH, United States, 7Imaging Research Core, Case Western Reserve University, Cleveland, OH, United States, 8Cancer Imaging Program, Case Western Reserve University, Cleveland, OH, United States, 9Department of Physiology and Biophysics, Case Western Reserve University, Cleveland, OH, United States This study compared the transport of three MRI contrast agents with different molecular sizes (Gd-DTPA, GadoSpin, and H217O) in the mouse glymphatic system with dynamic contrast-enhanced MRI. Dynamic signal changes in the whole-brain and selected regions of interest (ROI) were analyzed. The results showed drastically different kinetics of the three tracers, indicating differences in glymphatic transport routes and rates. The current work lays the foundation for future studies employing genetically manipulated mouse models for mechanistic understanding of the regulation of glymphatic function in normal and diseased conditions. |
|
| 17:45 | 0325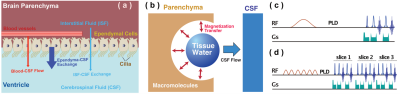 |
Age dependent cerebrospinal fluid-tissue water exchange detected by non-invasive magnetization transfer indirect spin labeling MRI
Anna Li1, Chen Lin1,2, Hongshuai Liu3, Yuguo Li1,4, Wenzhen Duan3, and Jiadi Xu1,4
1F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Research Institute, Baltimore, MD, United States, 2Department of Electronic Science, Xiamen University, Xiamen, China, 3Division of Neurobiology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Our understanding of the CSF exchange with surrounding tissues and its correlation with brain function is limited. We presented a non-invasive magnetization transfer indirect spin labeling (MISL) MRI method to quantify the water exchange between cerebrospinal fluid (CSF) and surrounding tissues in the brain by making use of the significantly different protein concentrations between CSF and other tissues. Water in the surrounding tissue is directly labeled by magnetization transfer from the abundant macromolecule pool. The reduction in the CSF signal corresponds to the water traveled to CSF, which is strongly age-dependent and weakly AQP4-dependent.
|
|
| 17:57 | 0326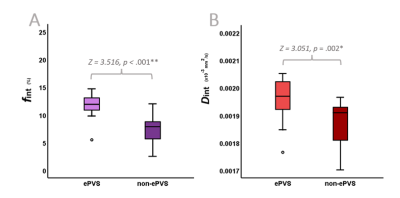 |
On the origin of a potential clearance marker: The contribution of enlarged perivascular fluid diffusion to a 7T IVIM interstitial fluid proxy
Merel M. van der Thiel1,2, Nathalie A. Roos1,3, Gerhard S. Drenthen1,2, Paulien H.M. Voorter1,2, Thorsten Feiweier4, Inez H.G.B. Ramakers2,5, Walter H. Backes1,2,6, and Jacobus F.A. Jansen1,2,7
1Department of Radiology & Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2School for Mental Health & Neuroscience, Maastricht University, Maastricht, Netherlands, 3Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 4Siemens Healthcare, Erlangen, Germany, 5Department of Psychiatry & Neuropsychology, Maastricht University, Maastricht, Netherlands, 6School for Cardiovascular Disease, Maastricht University, Maastricht, Netherlands, 7Department of Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands
Between the parenchymal and microvascular diffusion components, a third, intermediate component can be derived with cerebral IVIM. Its fraction (fint) seems a promising biomarker for altered cerebral clearance function. fint has been suggested to both be driven by fluid diffusivity within enlarged perivascular spaces (ePVS) and interstitial fluid between parenchymal cells. Using 7T MRI, this study determined a higher fint within the ePVS (through segmented masks) compared to surrounding non-ePVS tissue. Thereby, this study illustrates the contribution of ePVS fluid diffusivity to fint and highlights the ability to specifically assess ePVS fluid diffusion using a non-invasive MRI method.
|
|
| 18:09 | 0327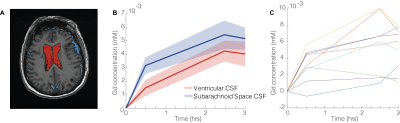 |
Quantitative imaging of brain to cerebrospinal fluid molecular clearance
Anders Wåhlin1,2,3, Anders Garpebring3, Klara Mogensen3, Tomas Vikner3, Cecilia Björnfot3, Sara Qvarlander3, Jan Malm4, and Anders Eklund2,3
1Department of Applied Physics and Electronics, Umeå University, Umeå, Sweden, 2Umeå Center for Functional Brain Imaging (UFBI), Umeå University, Umeå, Sweden, 3Department of Radiation Sciences, Umeå University, Umeå, Sweden, 4Department of Clinical Science, Umeå University, Umeå, Sweden
The pathways of brain molecular clearance and potential glymphatic efflux to extracerebral cerebrospinal fluid is a matter of intense research. We investigated possible contrast enrichment in cerebrospinal fluid of the subarachnoid space following intravenous contrast injection. This approach utilizes a subtle but widespread contrast leakage across the blood-brain barrier that potentially enables the investigation of glymphatic function. Using T1-mapping as well as dynamic scanning during contrast infusion, we present data consistent with a model where molecules cleared from the brain enter subarachnoid space cerebrospinal fluid. This approach could be a clinically feasible alternative for investigating brain molecular clearance in humans.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.