Power Pitch
Pitch: Microstructure from All Angles: Diffusion & Susceptibility
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

Power Pitch Session: How it Works
1st Hour: 2-minute Power Pitches in the Power Pitch Theater.
2nd Hour: 60-minute digital poster presentations at the smaller screens around the perimeter of the Power Pitch Theater.
| 17:00 | 0762.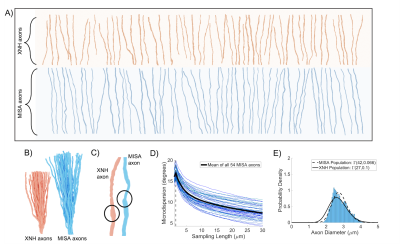 |
Microstructure-Informed Synthetic Axons (MISA): generating synthetic axons from 3D histology
Mariam Andersson1,2,3, Jonathan Rafael-Patino1,4,5, Hans Martin Martin Kjer2,3, Marco Pizzolato2,3,4, Gabriel Girard4,5,6, Jean-Philippe Thiran4,5,6, and Tim B. Dyrby2,3
1* (Equal contributions), Lausanne, Switzerland and Copenhagen, Denmark, 2Copenhagen University Hospital - Amager and Hvidovre, Danish Research Centre for Magnetic Resonance, Copenhagen, Denmark, 3Department of Applied Mathematics and Computer Science, Technical University of Denmark, Kgs. Lyngby, Denmark, 4Signal Processing Laboratory (LTS5), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 5Radiology Department, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Lausanne, Switzerland, 6CIBM Center for Biomedical Imaging, Lausanne, Switzerland
Changes in diffusion properties of the intra-axonal space can be linked to brain health and function. Monte Carlo simulations of diffusion using realistic substrates are important for sequence design and for understanding diffusion MRI signals observed ex vivo and in vivo. However, it is difficult to construct tissue phantoms that accurately reflect compartment morphology. Here, we present the framework MISA for the generation of Microstructure-Informed Synthetic Axons. MISA uses statistical descriptions of axonal morphology from 3D histology to mimic real axons. We show here that MISA axons match axons from 3D histology in terms of their diffusion properties.
|
|
| 17:02 | 0763.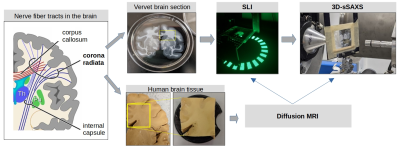 |
Towards a gold standard for fiber orientations in the brain: Validating dMRI using scattered light and X-ray imaging
Marios Georgiadis1, Miriam Menzel2, David Gräßel2, Ivan Rajkovic3, Donald Born1, Markus Axer2, and Michael Zeineh1
1Stanford University School of Medicine, Stanford, CA, United States, 2Institute of Neuroscience and Medicine (INM-1), Forschungszentrum Jülich, Julich, Germany, 3Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, Menlo Park, CA, United States
Generating a detailed network model of the brain requires a correct mapping of fiber orientations. Diffusion MRI is sensitive to neuronal alignment, yet each voxel contains hundreds of axons and other structures. Light and X-ray scattering can reveal more detailed information about nerve fibers, with much higher resolution and specificity respectively. Here we combine two methods, namely Scattered Light Imaging (SLI) and 3D-scanning small-angle X-ray scattering (3D-sSAXS), aiming to provide a micrometer-resolution gold standard for fiber orientation imaging. We compare that to high-resolution diffusion MRI of a region with challenging fiber orientations, the corona radiata, in human and non-human primates.
|
|
| 17:04 | 0764.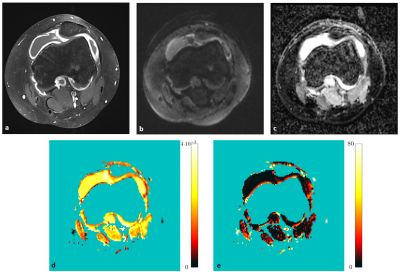 |
Intravoxel incoherent motion (IVIM) MRI for juvenile idiopathic arthritis in the knee: a prospective pilot study
Kilian Stumpf1, Britta Huch2, Anna-Katinka Bracher2, Thomas Hüfken1, Meinrad Beer2, Henning Neubauer2, and Volker Rasche1
1Department of Internal Medicine II, Ulm University Medical Center, Ulm, Germany, 2Department for Diagnostic and Interventional Radiology, Ulm University Medical Center, Ulm, Germany
Intravoxel incoherent motion (IVIM) imaging allows the quantification of diffusion and pseudo-perfusion in tissues and has been proposed as an alternative to conventional contrast agent enhanced (CE) T1-weighted scans. Its qualitative benefits for diagnosing juvenile idiopathic arthritis in the knee has previously been demonstrated. In this work, quantitative data of diffusion and perfusion fraction values between synovitis patients and healthy volunteers were compared in a prospective setting.
|
|
17:06 |
0765.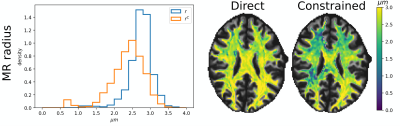 |
Axonal diffusivities from two-shell PGSE data
Marco Pizzolato1,2,3, Mariam Andersson3, Erick Jorge Canales-Rodríguez2, and Tim Bjørn Dyrby1,3
1Department of Applied Mathematics and Computer Science, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Signal Processing Lab (LTS5), École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 3Danish Research Centre for Magnetic Resonance, Copenhagen University Hospital - Amager and Hvidovre, Copenhagen, Denmark
The strongly diffusion-weighted MRI signal contains information about the axonal parallel and perpendicular diffusivities. Powder averaging has simplified the estimation of the perpendicular diffusivity, however it is difficult to estimate the parallel one because, as we show, the powder-averaged signal at strong diffusion weightings is insensitive to it. In this work we propose a method that enables the estimation of both diffusivities from only two conventional linear PGSE signal shells collected at high b-values. The method is tested on public MGH-HCP data to retrieve axonal diffusivities and calculate the MR radius in white matter.
|
|
| 17:08 | 0766.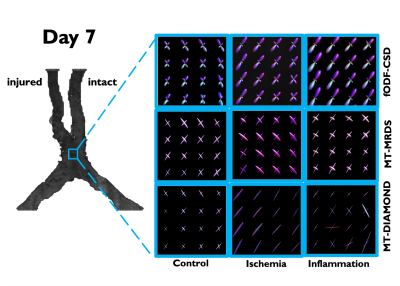 |
Longitudinal evaluation of bundle-wise diffusion metrics in a region of fiber crossing for axonal degeneration and inflammation
Ricardo Coronado-Leija1, Omar Narvaez2, Ricardo Rios3, Benoit Scherrer4, Alonso Ramirez-Manzanares5, and Luis Concha3
1Radiology, New York University School of Medicine, New York, NY, United States, 2University of Eastern Finland, Kuopio, Finland, 3Institute of Neurobiology, Universidad Nacional Autonoma de Mexico, Queretaro, Mexico, 4Boston Children's Hospital, Harvard Medical School, Boston, MA, United States, 5Center of Research in Mathematics, Guanajuato, Mexico
Single fiber methods, such as the diffusion tensor, cannot accurately determine per-bundle characteristics in voxels occupied by multiple axonal populations specially in the case of pathology when they have different diffusion properties. In this work, we used animal models of axonal degeneration and inflammation to evaluate longitudinally the sensitivity of bundle specific metrics provided by three multi-fiber methods in a region of crossing fiber in which only one fiber population is injured.
|
|
| 17:10 | 0767.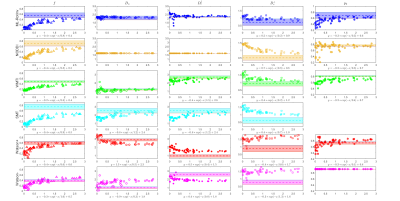 |
Comparison of diffusion MRI models for white matter in simulations and in early human brain development
Ying Liao1, Santiago Coelho1, Jenny Chen1, Benjamin Ades-Aron1, Dmitry S. Novikov1, and Els Fieremans1
1Radiology, NYU School of Medicine, New York, NY, United States
Biophysical modeling of diffusion MRI is instrumental in achieving specificity to tissue microstructure in human white matter. The “Standard Model” (SM) framework encompasses many approaches assuming multiple Gaussian compartments. To robustly estimate SM parameters, different constraints and techniques have been applied, resulting in different outcomes. Here we evaluate the precision and accuracy of commonly used implementations and constraints for SM parameter estimation, and compare their results both in simulations and in early human brain development.
|
|
| 17:12 | 0768.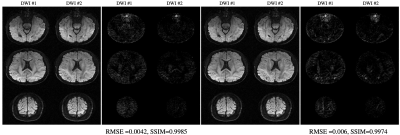 |
Rapid Multi-shell Diffusion MRI Enabled by Learning Compact and Rotation Invariant q-Space Representations
Merry Mani1, Baolian Yang2, Vincent Magnotta1, and Mathews Jacob1
1University of Iowa, Iowa City, IA, United States, 2GE Healthcare, Waukesha, WI, United States
A modified reconstruction is proposed for highly accelerated dMRI. The method employs machine learning in a model-based setting. The current work improves the generalizability of the deep-learned plug-and-play prior employed in the reconstruction, for enabling utilization of the reconstruction from a wide range of acquisition settings. This is achieved by learning a compact q-space representation in a rotation invariant space. The method is tested for the reconstruction of combined multi-band and in-plane accelerated data from both single-shell and multi-shell experiments. The reconstruction error is shown to be less than 3% for net acceleration of R=12 for single- and multi-shell cases.
|
|
| 17:14 | 0769.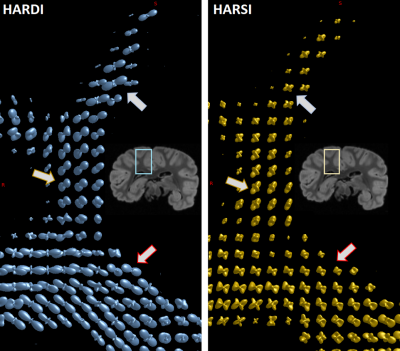 |
Beyond DW-Based Analysis of Fiber Architecture: Estimating Orientation Distributions from High Angular Resolution Susceptibility Imaging
Dimitrios G. Gkotsoulias1, Michael Paquette1, Cornelius Eichner1, Roland Müller 1, Torsten Schlumm1, Niklas Alsleben1, Jingjia Chen2, Carsten Jäger1, Jennifer Jaffe3,4, André Pampel1, Catherine Crockford3,4, Roman Wittig3,4, Alfred Anwander1, Chunlei Liu2, and Harald Möller1
1Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Department of Electrical Engineering and Computer Sciences, UC Berkeley, Berkeley, CA, United States, 3Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany, 4Tai Chimpanzee Project, Centre Suisse de Recherches Scientifiques en Cote d'Ivoire, Abidjan, Cote D'ivoire
We present a novel approach to estimate fiber Orientation Distribution Functions (ODFs) by applying the generalized Constant Solid Angle (CSA) method to High Angular Resolution Susceptibility Imaging (HARSI) data from post-mortem chimpanzee brain. The acquisition details and analytical pipelines are presented and derived susceptibility tensor metrics and ODFs are compared to metrics derived from traditional High Angular Resolution Diffusion Imaging (HARDI). The ODFs estimated from susceptibility data indicate comparable efficiency in resolving intersecting fiber orientations compared to HARDI-ODFs and increased sensitivity to secondary direction. This suggests a potential to obtain complementary information on brain white matter microstructural properties.
|
|
17:16 |
0770.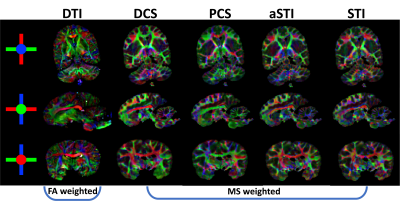 |
DECOMPOSE-STI: decompose sub-voxel diamagnetic and paramagnetic susceptibility tensors
Jingjia Chen1, Dimitrios Gkotsoulias2, Jennifer Jaffe3,4, Catherine Crockford3,4, Roman Wittig3,4, Harald E. Möller2, and Chunlei Liu1,5
1Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States, 2Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany, 4Taï Chimpanzee Project, Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Cote D'ivoire, 5Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, CA, United States
We investigate susceptibility tensor imaging (STI) reconstruction by separating the diamagnetic from paramagnetic susceptibility tensors using the recently introduced DECOMPOSE method. The resulting para-/diamagnetic susceptibility tensors produce spatially more coherent eigenvectors than conventional STI and it shows the potential to reduce the number of orientations needed for STI.
|
|
| 17:18 | 0771.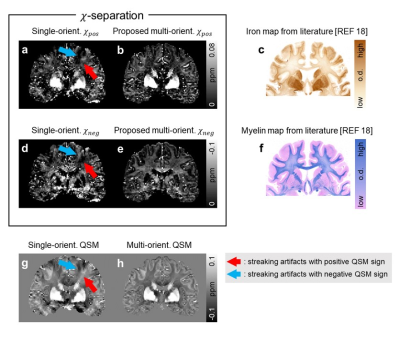 |
chi-separation using multi-orientation data in invivo and exvivo brains: Visualization of histology up to the resolution of 350 μm
Hyeong-Geol Shin1, Jincheol Seo2, Youngjeon Lee2, Hwihun Jeong1, Sooyeon Ji1, Minjun Kim1, Jang Woo Park3, Byeong C. Kim4, Kyung-Hwa Lee5, Seong Heon Kim6, Jaewon Jang6, Myung Kyun Woo1, and Jongho Lee1
1Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea, Republic of, 2National Primate Research Center, Cheongju, Korea, Republic of, 3Korea Radioisotope center for pharmaceuticals, Seoul, Korea, Republic of, 4Chonnam National University Medical School, Gwangju, Korea, Republic of, 5Chonnam National University Hwasun Hospital and Medical School, Gwangju, Korea, Republic of, 6Kangwon National University Hospital, Chuncheon, Korea, Republic of
Recently, the susceptibility source separation method, χ-separation, was suggested to separate paramagnetic and diamagnetic susceptibility distributions in the brain, potentially providing quantitative information of paramagnetic iron and diamagnetic myelin. However, the ill-posed nature of dipole-inversion has hindered accurate estimation and direct comparison with histology. Here, we extended the model for multi-orientation GRE, resolving the ill-posedness. The new model is applied to in-vivo and ex-vivo, revealing exquisite details of susceptibility distribution. When the results are compared to iron and myelin histology, great similarities are observed, suggesting the potentials of χ-separation for non-invasively acquiring three-dimensional histological information of iron and myelin.
|
|
| 17:20 | 0772.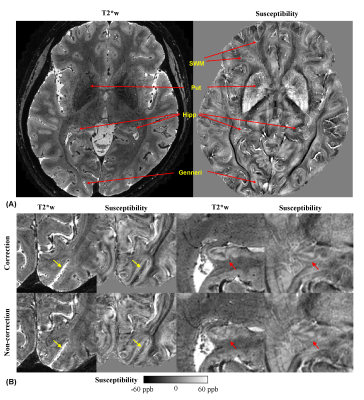 |
In vivo quantitative laminar R2* and susceptibility imaging at 0.3 mm in-plane resolution
Jiaen Liu1, Peter van Gelderen2, Xu Li3,4, Jacco A. de Zwart2, Kuo-Wei Lai4,5, Jeremias Sulam5, Erin S. Beck6, Serhat V. Okar2, Peter C.M. van Zijl3,4, Daniel S. Reich2, and Jeff H. Duyn2
1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 3Department of Radiology and Radiological Sciences, Johns Hopkins University, Baltimore, MD, United States, 4F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 5Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD, United States, 6Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
High resolution submillimeter MRI of myelin and iron may enhance our capability to define neural diseases more accurately and at an earlier stage. Susceptibility-weighted MRI methods are intrinsically sensitive to myelin and iron and benefit from the increased contrast-to-noise ratio at 7 T. In this study, we systematically investigated R2* and susceptibility distributions in cortical layers of healthy subjects with 0.3 mm in-plane resolution and 0.4 mm slice thickness. This effort was facilitated by a robust navigator-based motion- and B0-corrected GRE sequence.
|
|
| 17:22 | 0773.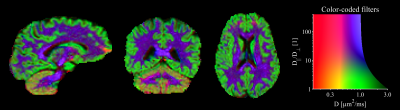 |
Diffusion-filtered imaging: Towards tissue-specific diffusion-MRI contrasts by combinations of tensor-valued diffusion encoding
Filip Szczepankiewicz1, Geraline Vis1, Danielle van Westen1, Carl-Fredrik Westin2, Pia M Sundgren1, and Markus Nilsson1
1Clinical Sciences Lund, Lund University, Lund, Sweden, 2Radiology, Brigham and Women's Hospital, Boston, MA, United States
Diffusion weighted imaging has been used both as a radiological tool that provides a simple biomarker without quantitative qualities, and more recent efforts have endeavored to make it quantitative by fitting of biophysical models or representations. In this work, we explore the novel radiological contrasts that can be generated by introducing tensor-valued diffusion encoding. Unlike most model-based approaches, these contrasts can be produced by rapid acquisition schemes and they produce novel contrasts that may contribute new diagnostic and radiological biomarkers.
|
|
| 17:24 | 0774.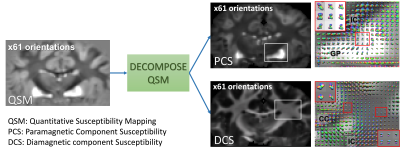 |
Resolve fiber crossings using orientation distribution function (ODF) of decomposed sub-voxel paramagnetic and diamagnetic susceptibility
Jingjia Chen1, Dimitrios Gkotsoulias2, Jennifer Jaffe3,4, Catherine Crockford3,4, Roman Wittig3,4, Harald E. Möller2, and Chunlei Liu1,5
1Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States, 2Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany, 4Taï Chimpanzee Project, Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Abidjan, Cote D'ivoire, 5Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, CA, United States
DECOMPOSE-QSM was applied on high angular-resolution multi-echo gradient-echo data of a chimpanzee brain to evaluate its ability in resolving orientation mixture of substructure fiber bundles. The orientation distribution function (ODF) of the separated paramagnetic and diamagnetic susceptibility provides smooth transitions of multiple fiber orientations. Additionally, the paramagnetic maps from DECOMPOSE show a potential to resolve fiber crossings in deep gray matter regions.
|
|
| 17:26 | 0775.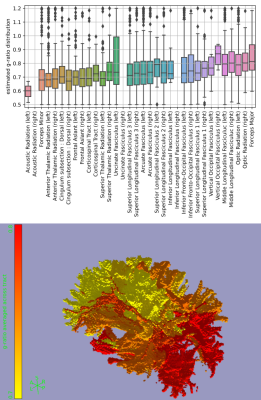 |
In vivo myelin thickness estimates from DIffusion-Prepared Phase Imaging (DIPPI)
Michiel Cottaar1, Wenchuan Wu1, Karla Miller1, and Saad Jbabdi1
1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
DIPPI is a novel sequence that allows the measurement of the off-resonance field experienced by intra-axonal water, which in myelinated axons is modulated by the surrounding myealin sheath allowing for estimation of the g-ratio. Unlike other g-ratio imaging methods, DIPPI does not rely on combining multiple modalities and can provide g-ratio estimates for individual crossing fibre populations rather than averaged over voxels. We present the first in-vivo DIPPI measurements and correct it for confounds such as subject movement and eddy currents. We then estimate the g-ratio in multiple white matter tracts from the processed off-resonance field maps.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.