Power Pitch
Pitch: Multiple Sclerosis
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

Power Pitch Session: How it Works
1st Hour: 2-minute Power Pitches in the Power Pitch Theater.
2nd Hour: 60-minute digital poster presentations at the smaller screens around the perimeter of the Power Pitch Theater.
| 09:15 | 0197.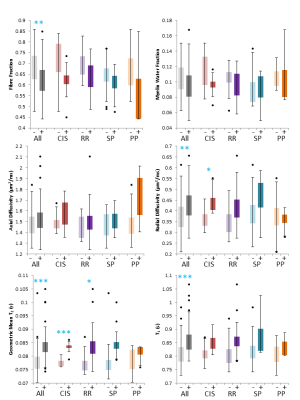 |
Diffusely abnormal white matter microstructural changes are similar across the clinical phenotypes of multiple sclerosis
Irene Margaret Vavasour1,2, Pierre Becquart1, Tigris Joseph1,2, Jasmine Gill1, Guojun Zhao3, Farhia Abdullahi1, Emily Kamma1, Kaya Frese1, Robert Carruthers1, Shannon H Kolind1,2,3, Alice Schabas1, Ana-Luiza Sayao1, Virginia Devonshire1, Roger Tam1,3, GR Wayne Moore1,2, Sophie Stukas1, Cheryl Wellington1, Jacqueline Quandt1, Anthony Traboulsee1,3, David Li1,3,
and Cornelia Laule1,2
1University of British Columbia, Vancouver, BC, Canada, 2International Collaboration on Repair Discoveries, Vancouver, BC, Canada, 3MSMRI Research Group, Vancouver, BC, Canada
Diffusely abnormal white matter (DAWM) is commonly found on conventional brain MRI in all stages of multiple sclerosis (MS). Blood markers associated with prior or ongoing myelin and axonal damage were not found to be elevated in people with DAWM relative to people without DAWM. Within areas of DAWM, no differences between MS phenotypes were found possibly suggesting that the tissue abnormalities within DAWM are similar across the clinical course, from early to late stages of MS.
|
|
| 09:17 | 0198.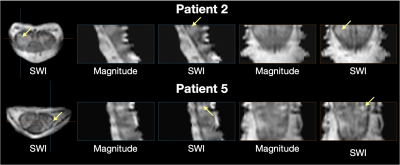 |
In pursuit of the central vein sign in multiple sclerosis in the cervical spinal cord at 7T
Ryan K Robison1,2,3, Atlee A Witt4, Anna JE Combes2,3, Kristin P O'Grady2,3, Logan E Prock3, Delaney Houston5, Dann C Martin5, Margareta Clarke5, and Seth A Smith2,3
1Philips, Nashville, TN, United States, 2Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 3Vanderbilt University Institute of Imaging Sciences, Nashville, TN, United States, 4Vanderbilt University School of Medicine, Nashville, TN, United States, 5Vanderbilt University Medical Center, Nashville, TN, United States
Central vein sign is believed to be a specific marker for the diagnosis of multiple sclerosis (MS) but has not yet been reported in the spine. This work used SWI in the cervical spinal cord of MS patients and healthy controls at 7T to look for evidence of central veins. SWI hypointensities were observed frequently around the cord periphery, between the white and grey matter within the cord, and along nerve roots. Hypointensities were also observed within spinal cord lesions, providing candidate evidence of central veins in lesions of the spinal cord.
|
|
| 09:19 | 0199.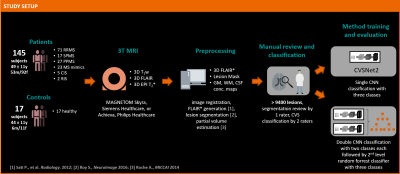 |
Improved Automated Central Vein Sign Assessment by Multi-Level Classification
Till Huelnhagen1,2,3, Omar al Louzi4,5, Lynn Daboul4, Jonas Richiardi2, Daniel S. Reich4, Tobias Kober1,2,3, and Pascal Sati5
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 2Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 3Signal Processing Laboratory (LTS 5), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 4Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH), Bethesda, MD, United States, 5Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Central vein sign (CVS) assessment has shown potential to improve differential diagnosis in multiple sclerosis, but automating this task remains non-trivial. As human inter-rater agreement was reported to improve by separating the tasks of lesion exclusion and CVS assessment, we hypothesized that this could also benefit automated CVS assessment. To test this hypothesis, we implemented a novel multi-level classifier for automated CVS assessment and trained and evaluated it in more than 9400 expert-reviewed lesions. The new approach outperforms previous methods, achieving per-class accuracies of 76%–83% in an unseen testing set and >90% accuracy to identify MS cases.
|
|
| 09:21 | 0200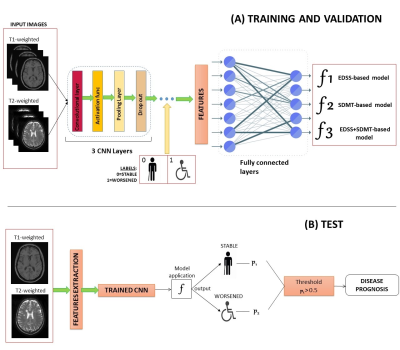 |
A Deep-Learning Approach to Predicting Disease Progression in Multiple Sclerosis Using Magnetic Resonance Imaging Video Permission Withheld
Loredana Storelli1, Matteo Azzimonti1,2, Mor Gueye1,2, Paolo Preziosa1,2, Carmen Vizzino1, Gioacchino Tedeschi3, Nicola De Stefano4, Patrizia Pantano5,6, Massimo Filippi1,2,7,8,9, and Maria A. Rocca1,2,9
1Neuroimaging Research Unit, Division of Neuroscience, IRCCS San Raffaele Scientific Institute, Milan, Italy, 2Neurology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy, 3Department of Advanced Medical and Surgical Sciences, and 3T MRI-Center, University of Campania “Luigi Vanvitelli”, Maples, Italy, 4Department of Medicine, Surgery and Neuroscience, University of Siena, Siena, Italy, 5Department of Human Neurosciences, Sapienza University of Rome, Rome, Italy, 6IRCCS NEUROMED, Pozzilli, Italy, 7Neurorehabilitation Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy, 8Neurophysiology Service, IRCCS San Raffaele Scientific Institute, Milan, Italy, 9Vita-Salute San Raffaele University, Milan, Italy
Artificial intelligence (AI) approaches have been applied in several fields of multiple sclerosis (MS) in recent years. However, their application to predict disease progression remains largely unexplored. In this study, we obtained a robust and accurate AI tool for predicting clinical and cognitive evolution at two years for MS patients, based on just T1-weighted and T2-weighted brain MRI scans at baseline visit, which exceeded the performance of two expert physicians blinded to patients’ clinical history. This algorithm has the potential to be an important tool to support physicians for a prompt recognition of MS patients at risk of disease worsening.
|
|
| 09:23 | 0201.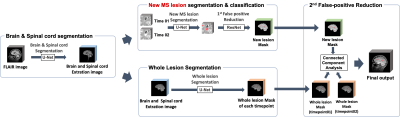 |
A Multi-Stage 3D Patch-wise Deep Learning Algorithm for Detection of New Multiple Sclerosis Lesions in Longitudinal MRI
Junghwa Kang1, Siyun Jung1, Jeongmin Yim2, Jinhee Jang3, and Yoonho Nam1
1Division of biomedical engineering, Hankuk University of Foreign Studies, gyunggi-do,yongin-si, Korea, Republic of, 2College of Medicine, Catholic University of Korea, Seoul, Korea, Republic of, 3Department of Radiology, Seoul St. Mary's Hospital, Seoul, Korea, Republic of
Detecting changes in multiple sclerosis (MS) lesions through follow-up MR images is an important but time-consuming and subjective process. In this work, we propose a fully automatic deep learning-based method to detect new MS lesions. The model was trained and tuned using the MSSEG2 challenge dataset. First, the brain and spinal cord masks were generated, and registration between two time points was performed. Then, new lesions and whole lesions were segmented by patch-wise inputs, respectively. The final mask for new lesions was produced by comparing these two segmentations, and in this way we could effectively reduce false-positives.
|
|
| 09:25 | 0202.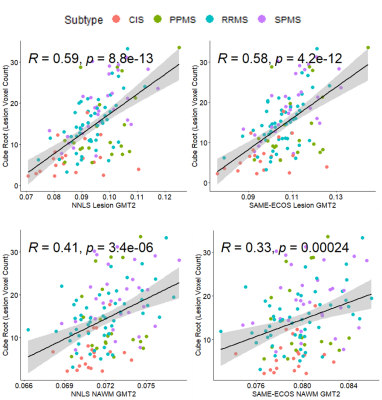 |
Multiple sclerosis T2 lesion volume correlates weakly with myelin water fraction
Tigris S. Joseph1,2, Hanwen Liu2,3,4, Guojun Zhao4, Shannon H. Kolind1,2,4,5, Robert Carruthers4, Alice Schabas4, Ana-Luiza Sayao4, Virginia Devonshire4, Roger Tam5,6, G. R. Wayne Moore2,4,7, David K. B. Li4,5, Anthony Traboulsee4, Irene M. Vavasour2,5, and Cornelia Laule1,2,5,7
1Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 2International Collaboration on Repair Discoveries, University of British Columbia, Vancouver, BC, Canada, 3Montreal Neurological Institute - Hospital, McGill University, Montreal, QC, Canada, 4Medicine, University of British Columbia, Vancouver, BC, Canada, 5Radiology, University of British Columbia, Vancouver, BC, Canada, 6School of Biomedical Engineering, University of British Columbia, Vancouver, BC, Canada, 7Pathology & Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada
Multiple sclerosis (MS) lesion volume is commonly reported but is not pathologically specific or strongly associated with MS disability. Lesions are not necessarily demyelinated, which may be why lesion volume and disability are not strongly correlated. MS lesion volume was compared to Myelin Water Imaging (MWI) derived metrics related to myelin content (myelin water fraction) and inflammation (geometric mean T2) in MS normal appearing white matter and lesions. Weak correlations were observed between lesion volume and MWF, highlighting that the amount of lesional tissue does not reflect the degree of myelin damage within the brain.
|
|
| 09:27 | 0203.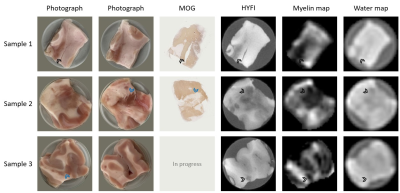 |
Mapping the myelin bilayer with short-T2 MRI: Application to ex-vivo multiple sclerosis brain tissue
Emily Louise Baadsvik1, Markus Weiger1, Romain Froidevaux1, Wolfgang Faigle2, Benjamin Victor Ineichen3, and Klaas Paul Pruessmann1
1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zurich, Switzerland, 2Neuroimmunology and MS Research Section, Neurology Clinic, University of Zurich, University Hospital Zurich, Zurich, Switzerland, 3Department of Neuroradiology, Clinical Neuroscience Center, University Hospital Zurich, University of Zurich, Zurich, Switzerland
Signal from the myelin bilayer can be captured using dedicated short-T2 imaging techniques, and analysis methods to isolate the contribution of the bilayer from other signal sources have been proposed. However, such analysis methods are often validated on samples in which the background water signal has been suppressed. Thus, it is unclear whether these techniques can successfully map myelin content in a clinical setting. Here, we apply and evaluate a previously proposed technique for myelin mapping to multiple sclerosis brain tissue without water suppression. We conclude that the quality of the myelin maps is comparable to that of water-suppressed samples.
|
|
| 09:29 | 0204.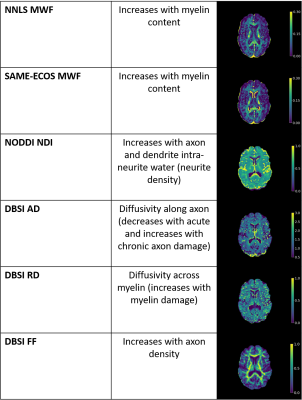 |
Myelin water and multi-shell diffusion imaging provide unique information about multiple sclerosis myelin and axonal damage
Tigris S. Joseph1,2, Hanwen Liu2,3,4, Shannon H. Kolind1,2,4,5, Guojun Zhao4, Peng Sun6, Robert Carruthers4, Alice Schabas4, Ana-Luiza Sayao4, Virginia Devonshire4, Roger Tam5,7, G. R. Wayne Moore2,4,8, David K. B. Li4,5, Sheng-Kwei Song9, Anthony Traboulsee4, Irene M. Vavasour2,5, and Cornelia Laule1,2,5,8
1Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 2International Collaboration on Repair Discoveries, University of British Columbia, Vancouver, BC, Canada, 3Montreal Neurological Institute - Hospital, McGill University, Montreal, QC, Canada, 4Medicine, University of British Columbia, Vancouver, BC, Canada, 5Radiology, University of British Columbia, Vancouver, BC, Canada, 6Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States, 7School of Biomedical Engineering, University of British Columbia, Vancouver, BC, Canada, 8Pathology & Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada, 9Radiology, Washington University, St. Louis, MO, United States
Myelin Water Imaging (MWI), Diffusion Basis Spectrum Imaging (DBSI) and Neurite Orientation Dispersion and Density Imaging (NODDI) were used to assessed relationships between myelin and axon-related measures in 122 multiple sclerosis (MS) participants and 16 healthy controls. Neurite density index (NDI) correlated strongly with radial diffusivity and weakly with myelin water fraction, suggesting that radial diffusivity also captures diffusion in dendrites. The lack of correlation between NDI and fiber fraction was surprising given that both metrics are meant to relate to axon density. MWI, DBSI and NODDI provide unique and complementary information about MS damage.
|
|
| 09:31 | 0205.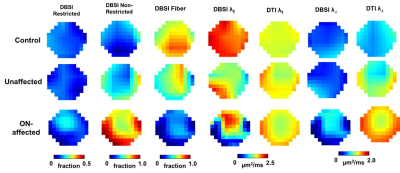 |
Imaging Optic Nerve Pathology and Dysfunction in Multiple Sclerosis Using Diffusion Basis Spectrum imaging
Tsen-Hsuan (Abby) Lin1, William M Spees1, Michael Wallendorf2, Peng Sun1,3, Junqian Xu4, Anne H Cross5,6, and Sheng-Kwei Song1,6
1Radiology, Washington University School of Medicine, St. Louis, MO, United States, 2Biostatistics, Washington University School of Medicine, St. Louis, MO, United States, 3Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States, 4Radiology, Baylor College of Medicine, Houston, TX, United States, 5Neurology, Washington Univeristy School of Medicine, St. Louis, MO, United States, 6Hope Center for Neurological Disorders, Washington University School of Medicine, St. Louis, MO, United States
We have introduced diffusion basis spectrum imaging (DBSI) to detect, differentiate, and quantify coexisting pathologies in people with multiple sclerosis (MS). Recently, we performed functional DBSI and DTI with flashing-checkerboard stimulation. DBSI-derived radial diffusivity (DBSI-RD) decreased significantly during visual stimulation while DTI-RD did not change. In this study, we employed fDBSI to assess optic nerve function and pathology simultaneously in MS. Axonal loss and vasogenic edema/increased extracellular space attenuated optic nerve response to visual stimulation.
|
|
| 09:33 | 0206.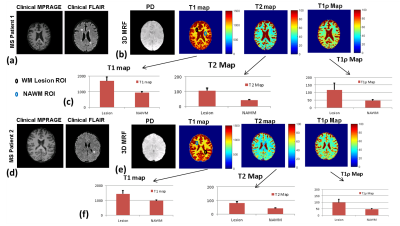 |
Three dimensional multi-parameter brain mapping using MR fingerprinting for characterization of multiple sclerosis lesions
Rajiv G Menon1, Azadeh Sharafi1, Marco Muccio1, Tyler E. Smith2, Ilya Kister2, Yulin Ge1, and Ravinder R Regatte1
1Center for Biomedical Imaging, Grossman school of Medicine, NYU Langone Health, New York, NY, United States, 2Department of Neurology, Grossman School of Medicine, NYU Langone Health, New York, NY, United States
A 3D multi-parameter MRF imaging technique capable of quantifying 4 different parameters (T1, T2, T1ρ, B1) was developed for multiple sclerosis(MS) application. The 3D-MRF technique was tested with multiple shots on a standardized NIST/ISMRM phantom, and brain imaging in 5 healthy volunteers. The 3D-MRF was also tested on two MS patients with chronic and enhancing MS lesions. The phantom studies demonstrate that quantitative maps are in agreement with reference, healthy volunteer studies show the agreement between multiple shots and SNR improvement with increasing shots. The MS patient results demonstrate the potential use of mutli-parameter quantitative mapping for WM characterization.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.