Digital Poster
Artefacts
ISMRM & ISMRT Annual Meeting & Exhibition • 03-08 June 2023 • Toronto, ON, Canada

| Computer # | |||
|---|---|---|---|
3409.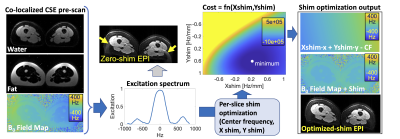 |
1 |
Slice-by-slice Dynamic Shimming Based on a Chemical
Shift-Encoded Acquisition to Improve Fat Suppression in DWI
Aidan Tollefson1,2,
Gaohong Wu3,
Patricia Lan4,
Arnaud Guidon5,
Rianne A Van Der Heijden2,
Daiki Tamada2,
Ali Pirasteh1,2,
and Diego Hernando1,2
1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3GE Healthcare, Waukesha, WI, United States, 4GE Healthcare, Menlo Park, CA, United States, 5GE Healthcare, Boston, MA, United States Keywords: Artifacts, Fat B0 inhomogeneities can lead to failures in chemical shift-based fat suppression, particularly in complex susceptibility environments. Conventional volumetric shimming methods are frequently unable to compensate for these B0 inhomogeneities, which leads to residual fat signal and artifacts in various applications. We propose a slice-by-slice shimming method that relies on information (water-only image, fat-only image, B0 fieldmap) derived from a rapid chemical shift-encoded acquisition. This method demonstrated improved fat suppression in diffusion weighted imaging (DWI) of the upper leg and may lead to improved reliability in other applications. |
|
3410.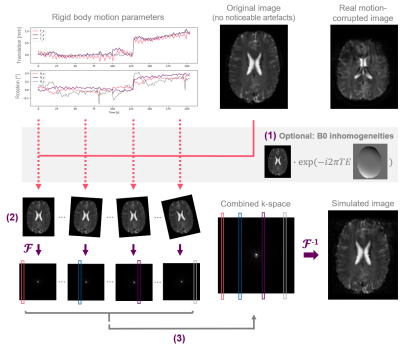 |
2 |
Investigating the Impact of Motion and Associated B0 Changes on
Oxygenation Sensitive MRI through Realistic Simulations
Hannah Eichhorn1,2,
Kerstin Hammernik3,4,
Samira M. Epp5,6,
Dimitrios C. Karampinos7,
Julia A. Schnabel1,2,8,
and Christine Preibisch9
1Institute of Machine Learning in Biomedical Imaging, Helmholtz Center Munich, Munich, Germany, 2TUM School of Computation, Information and Technology; and TUM Institute for Advanced Study, Technical University of Munich, Munich, Germany, 3Lab for Artificial Intelligence in Healthcare and Medicine, Technical University of Munich, Munich, Germany, 4Department of Computing, Imperial College London, London, United Kingdom, 5Department of Neuroradiology, Neuroimaging Center, Technical University of Munich, Munich, Germany, 6Graduate School of Systemic Neurosciences, Ludwig-Maximilians-University, Munich, Germany, 7Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 8School of Biomedical Imaging and Imaging Sciences, King’s College London, London, United Kingdom, 9Department of Neuroradiology, School of Medicine, Technical University of Munich, Munich, Germany Keywords: Artifacts, Oxygenation, Motion Simulation T2*-weighted gradient echo imaging is strongly impacted by subject head motion, amongst others due to motion-related changes in B0 inhomogeneities. Within the oxygenation-sensitive mqBOLD protocol, motion artifacts in T2*-weighted images lead to errors in derived parameter maps. To quantify these errors, we performed realistic motion simulations incorporating rigid body transformations and motion-related field inhomogeneity changes. Our results demonstrate the importance of including B0 inhomogeneities for realistic motion artifact patterns. Even small amounts of simulated motion resulted in substantial errors in derived T2* and R2’ parameter maps, which highlights the relevance of T2* motion correction within the mqBOLD technique. |
|
3411.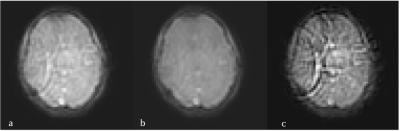 |
3 |
Independent Component Analysis for Noise Removal in MR
Fingerprinting
Emma L Thomson1,2,
Claudia A M Gandini Wheeler-Kingshott3,4,5,
and Geoff J M Parker1,6,7
1Centre for Medical Image Computing, Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 2UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, London, United Kingdom, 3NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, London, United Kingdom, 4Department of Brain & Behavioural Sciences, University of Pavia, Pavia, Italy, 5Brain Connectivity Centre Research Department, IRCCS Mondino Foundation, Pavia, Italy, 6NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, London, United Kingdom, 7Bioxydyn Limited, Manchester, United Kingdom Keywords: Artifacts, MR Fingerprinting We propose the use of independent component analysis for the removal of coherent noise sources prior to matching for magnetic resonance fingerprinting (MRF). We tested this technique for the removal of reconstruction artefacts on images acquired with a spiral k-space acquisition to quantify intravascular T1, extravascular T1, B1+, cerebral blood volume (νb) and inter-vascular water exchange (1/τb). We demonstrate that removal of coherent noise sources in this way can improve the precision of measurements of parameters. |
|
3412.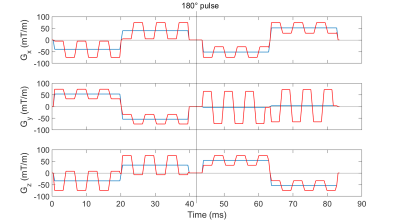 |
4 |
Signal gain by reduction of the concomitant phase in double
diffusion encoding by means of added oscillating gradients
Julian Rauch1,2,
Frederik B. Laun3,
Dominik Ludwig1,
Maxim Zaitsev4,
Mark E. Ladd1,2,5,
Peter Bachert1,2,
and Tristan A. Kuder1,2
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, Heidelberg University, Heidelberg, Germany, 3University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 4Medical Physics, Department of Radiology, Faculty of Medicine, Medical Center University of Freiburg, Freiburg, Germany, 5Faculty of Medicine, Heidelberg University, Heidelberg, Germany Keywords: Artifacts, Artifacts Intravoxel dephasing generated by Maxwell or concomitant fields can cause image artifacts like signal voids or falsify the quantification. In this study, an optimization scheme to reduce concomitant field effects in diffusion sequences with single pairs of bipolar gradients on each axis is presented. Oscillating gradients are added onto the original gradient pulses with the aim of reducing the concomitant phase without significant changes in the sequence properties. The proposed method is evaluated in both measurements and simulations, and gives rise to a positive effect on the signal for arbitrary diffusion wave vector pairs. |
|
3413.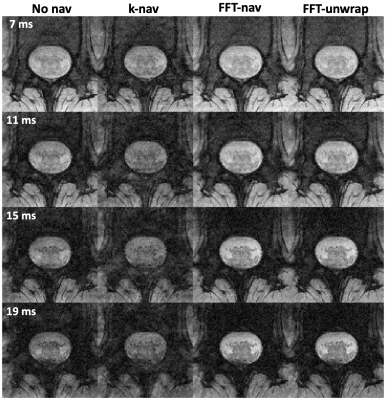 |
5 |
Optimised navigator correction of physiological field
fluctuations in multi-echo GRE of the lumbar spinal cord at 3T
Laura Beghini1,
Gergely David2,3,
Martina D. Liechti3,
Silvan Büeler3,
and S. Johanna Vannesjo1
1Department of Physics, Norwegian University of Science and Technology, Trondheim, Norway, 2Spinal Cord Injury Center, Balgrist University Hospital, University of Zurich, Zurich, Switzerland, 3Dept. of Neuro-Urology, Balgrist University Hospital, University of Zurich, Zurich, Switzerland Keywords: Artifacts, Spinal Cord Multi-echo GRE sequences acquired in the spinal cord are sensitive to breathing-related field variations, leading to ghosting artifacts. While navigator-based corrections are often used in the brain to account for field variations, standard implementations often fail in the spinal cord due to the close proximity to the lungs. Here, we investigated optimised navigator corrections including a fast Fourier transform combined with region selection encompassing the spinal canal. Besides a visual improvement of image quality, the optimised navigator correction yielded statistically significant improvements in the CNR between WM and CSF and the SNR in the WM. |
|
3414.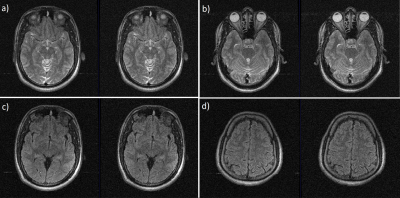 |
6 |
RF Interference Manifested Zipper Detection And Removal Using
Channel Compression
Megha Goel1,
Preetham Shankpal1,
Suresh Emmanuel Joel1,
Rajagopalan Sundareshan1,
and Harsh Agarwal1
1GE Healthcare, Bengaluru, India Keywords: Artifacts, Image Reconstruction, Zipper-removal Zipper artifacts are commonly seen in MR images due to spurious radio-frequency signals or improper RF-shielding. Zipper-riddled images lose diagnostic value and are usually sent for rescan. Here, we attempt to mitigate zipper artifacts in the post-processing pipeline after scan has been acquired. We do this after channel combination technique has limited zipper appearance to 1-2 pseudo-channels, which we detect and remove from channel-combination process. We evaluated this on various brain contrasts and confirm reduction of zipper presence visually in the images. Given that zippers manifest as bright/dark discontinuous lines irrespective of the anatomy/contrast, the method should be generalizable. |
|
3415.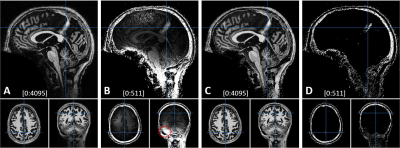 |
7 |
Denoising of 7T MP2RAGE MRI with Preserving the Brain Signal
Kwan-Jin Jung1
1Biomedical Imaging Center, Beckman Institute, University of Illinois at Urbana-Champaign, Urbana, IL, United States Keywords: Artifacts, High-Field MRI, MP2RAGE, 7T MP2RAGE requires denoising of the synthesized T1-weighted image. The conventional denoising method is to add a constant in the synthesis that alters the brain signal. This affects brain segmentation. A new denoising method has been developed to denoise it with preserving the brain signal. The new method estimates the bias field from two inversion images and sets the noise floors at the low and high noise levels in proportion to the bias field. The denoise weight is calculated at each voxel using the two noise floors. The proposed denoising method worked reliably with preserving the brain signal. |
|
3416.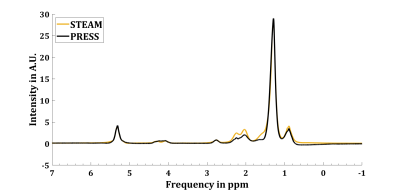 |
8 |
Effect of chemical shift displacement on Lipid Composition
determination in localized 1H NMR Spectroscopy
Julian Mevenkamp1,2,
Mijke Buitinga1,2,
Pandichelvam Veeraiah3,4,
and Vera B. Schrauwen-Hinderling1,2,5
1Nutrition & Movement Sciences, Maastricht University, Maastricht, Netherlands, 2Radiology & Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 3Scannexus, Maastricht, Netherlands, 4Faculty of Health Medicine & Life Sciences, Maastricht University, Maastricht, Netherlands, 5German Diabetes Center, Düsseldorf, Germany Keywords: Artifacts, Spectroscopy, Chemical Shift Displacement We showed that implementing prior knowledge about Chemical Shift Displacement Artifacts (CSDA) in localized MR-spectroscopy improves fit quality in the context of hepatic lipid composition measurements (LICO) performed with PRESS. Residuals of fitting PRESS spectra of peanut oil with CSDA prior knowledge show smaller residuals compared to assuming a purely Spin Echo based J-coupling evolution during the echo time. Furthermore, peanut oil LICO measured by PRESS and fitted with CSDA prior knowledge almost matched reference values determined by HR-NMR (PUFAHR-NMR = 23.5%, MUFAHR-NMR = 52.9%, SFAHR-NMR = 23.6% vs. PUFACSDA = 21.57%, MUFACSDA = 54.4%, SFACSDA = 24.0%). |
|
3417.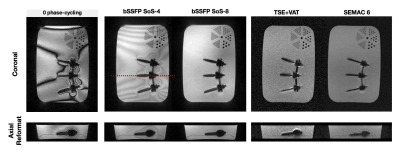 |
9 |
Isotropic sub-mm bSSFP imaging near metal at 0.55T
Kübra Keskin1,
Nam G. Lee2,
Jay Acharya3,
and Krishna S. Nayak1,2
1Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 3Radiology, University of Southern California, Los Angeles, CA, United States Keywords: Artifacts, Artifacts, Metal At conventional field strengths, the large magnetic susceptibility difference between metallic implants and surrounding tissues causes severe artifacts often requiring multi-spectral imaging. Recent low-field MRI systems offers new opportunities for imaging adjacent to metallic implants, including balanced SSFP. Here, we utilize phase-cycled bSTAR, a short-TR 3D dual-echo radial bSSFP sequence, for isotropic 3D banding artifact free imaging near metallic implants at 0.55T. We demonstrate diagnostic quality images in volunteers with wrist and spinal hardware with high SNR efficiency compared to TSE-based sequences. |
|
3418.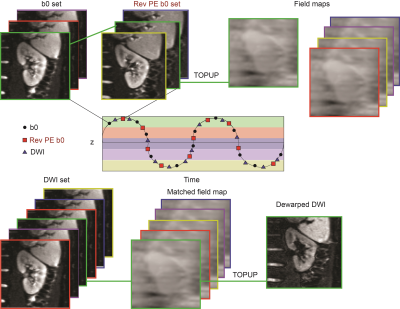 |
10 |
Demonstration of motion-dependent magnetic field inhomogeneity
in the kidneys and its retrospective correction for renal DWI
Nima Gilani1,
Artem Mikheev1,
Inge Manuela Brinkmann2,
Dibash Basukala1,
Thomas Benkert3,
Malika Kumbella1,
James S. Babb1,
Hersh Chandarana1,
and Eric E. Sigmund1
1Department of Radiology, NYU Langone Health, New York, NY, United States, 2Siemens Medical Solutions USA Inc., New York, NY, United States, 3Siemens Healthcare GmbH, Erlangen, Germany Keywords: Artifacts, Kidney, TOPUP, Field Inhomogeneity Echo planar imaging is highly affected by field map inhomogeneity distortion artifact. Field map inhomogeneity has shown to be motion dependent in the kidneys. In the present work, we propose an alternative method for correction of magnetic field inhomogeneity for renal DWI in respiratory-resolved fashion. Specifically, we collect a series of forward and reverse phase encoded b=0 images to sample kidney motion caused by breathing, map the spatial and respiratory phase dependence of the magnetic field inhomogeneity, and correct each image of free-breathing DWI series according to their respiratory phase. |
|
3419.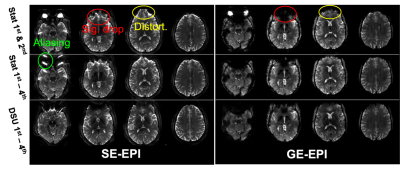 |
11 |
Investigation of Susceptibility Gradient Artifacts in EPI at 7T
Chan Hong Moon1,
Hoby Hetherington2,3,
and Jullie W. Pan2,4
1MRRC, Department of Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2NEXTGEN, Department of Radiology, University of Missouri, Columbia, MO, United States, 3Resonance Research, Inc., Billerica, MA, United States, 4University of Missouri, Columbia, MO, United States Keywords: Artifacts, High-Field MRI, EPI, 7T, Susceptibility, GRAPPA, Aliasing With the development of high-field 7T scanners, interest in high resolution neuroimaging for EPI-based functional and diffusion MRI has increased. However, EPI artifacts at 7T (spatial distortion and signal loss) increase compared to that seen at 3T due to increased B0 inhomogeneity. Furthermore, EPI artifacts become more significant and complicated when parallel imaging is used. As a result, 7T fMRI and DWI suffers from B0 inhomogeneity-induced aliasing as well as the more common EPI artifacts. In this study, the source of B0 inhomogeneity artifact in parallel imaging was investigated and methods to reduce these artifacts are proposed/tested at 7T. |
|
3420.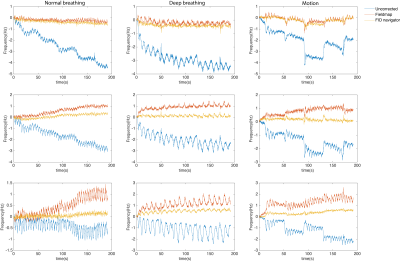 |
12 |
Spatiotemporal B0 correction in Oscillating Steady State Imaging
(OSSI) fMRI using Free Induction Decay Navigators (FIDnavs)
Mariama Salifu1,
Douglas Noll1,
and Melissa Haskell2,3
1Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States, 2Electrical Engineering, University of Michigan, Ann Arbor, MI, United States, 3Hyperfine Research, Guilford, CT, United States Keywords: Artifacts, Shims, B0 correction Temporal field variations (e.g., physiological noise) in oscillating steady state imaging (OSSI) not only increases signal fluctuations as seen in GRE, but also alters signal characteristics that lead to inconsistent k-space acquisitions from TR to TR. This effectively reduces the overall functional contrast and temporal signal to noise ratio (tSNR). Here, we utilized free induction decay (FID) signal phase to estimate up to the first-order field inhomogeneity coefficients for prospective spatiotemporal B0 field correction in OSSI. |
|
3421.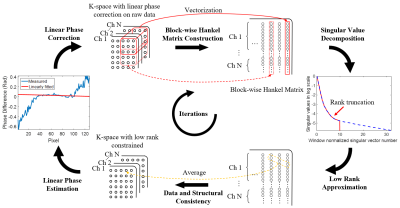 |
13 |
EPI Nyquist Ghost Correction by Iteratively Enforcing Structural
Low Rankness
Yilong Liu1,
Mengye Lyu2,
Yunlin Zhao1,
Linfang Xiao3,
and Ed X. Wu4,5
1Guangdong-Hongkong-Macau Institute of CNS Regeneration, Key Laboratory of CNS Regeneration (Ministry of Education), Jinan University, Guangzhou, China, 2College of Health Science and Environmental Engineering, Shenzhen Technology University, Shenzhen, China, 3Hangzhou Weiying Medical Technology Co., Ltd, Hangzhou, China, 4Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 5Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China Keywords: Artifacts, Artifacts, EPI, Nyquist ghost correction This study presents a Nyquist ghost correction method by iteratively enforcing low rankness of the block-wise Hankel matrix and updating the 1D linear model for phase correction. The proposed method was evaluated with multi-shot EPI data at both ultra-high field (7T) and low field (0.3T). Compared with existing SVD-based method, the proposed method requires much fewer iterations to achieve similar performance, provides a computationally efficient and robust solution to EPI Nyquist ghost correction. |
|
3422.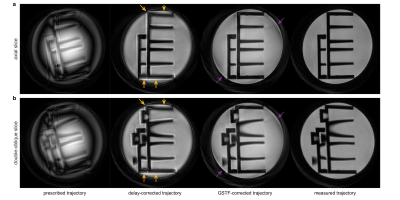 |
14 |
Preclinical spiral imaging with a high-performance gradient
insert: a comparison of trajectory correction methods
Hannah Scholten1,
Tobias Wech1,
Sascha Köhler2,
and Jürgen E. Schneider3
1Department of Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany, 2Bruker BioSpin MRI GmbH, Ettlingen, Germany, 3Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, United Kingdom Keywords: Artifacts, System Imperfections: Measurement & Correction, GIRF, GSTF, LTI We compared three different trajectory correction methods in spiral phantom images on a preclinical 7T scanner with a high-performance gradient insert. The first was an isotropic delay-correction, the second a gradient correction based on the gradient system transfer function (GSTF), and the third employed measured trajectories. In both an axial and a double-oblique slice, the measured trajectory resulted in the best image quality, while the delay-corrected images suffered from halo effects and signal bleeding. The GSTF-corrected images only had minor remaining artifacts, stemming from trajectory deviations due to non-linearities in the gradient chain. |
|
3423.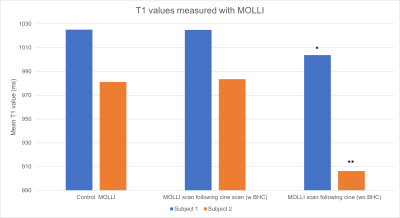 |
15 |
Cardiac T1 and T2 Mapping: The Importance of Rest Periods in
Quantitative Tissue Property Mapping
Seth Garrett, B.S.1,
Jesse Hamilton, Ph.D.1,
and Nicole Seiberlich, Ph.D.1
1Michigan Medicine- Department of Radiology, University of Michigan, Ann Arbor, MI, United States Keywords: Artifacts, Cardiovascular The aim of this study is to assess whether the presence of rest periods in the form of breathhold commands affects T1 and T2 values measured in the myocardium using both clinically standard pulse sequences and cardiac Magnetic Resonance Fingerprinting (MRF). When mapping data are collected immediately following cine acquisitions, T1 maps from both MOLLI and MRF show significant errors, although T2 maps from both T2-prepared bSSFP and MRF are unaffected by the absence of a pause between sequences. |
|
3424. |
16 |
Predicting MR Image Quality from Breathing Patterns
Soham S Vasanawala1 and
John M Pauly1
1Electrical Engineering, Stanford University, Stanford, CA, United States Keywords: Artifacts, Motion Correction, abdomen, respiration Motion, particularly from breathing, compromises the quality of magnetic resonance images. In this work, we hypothesize that detected breathing patterns can be utilized to predict whether adequate MR image quality will be obtained. With a K-means clustering algorithm, 9 in 10 forty-second breathing waveforms were correctly predicted as either resulting in a high or low image quality image; this finding can save time from unnecessary scans. Other models achieved similar results as K-means clusterings. |
|
3425.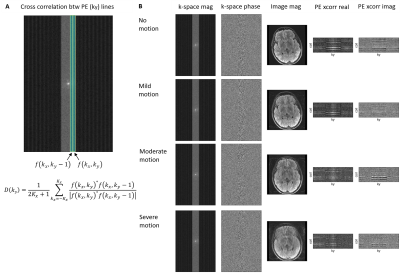 |
17 |
Clinical evaluation of k-space correlation informed motion
artifact detection in segmented multi-slice MRI
Ikbeom Jang1,2,
Malte Hoffmann1,2,
Nalini Singh3,4,
Yael Balbastre1,
Lina Chen5,
Marcio Aloisio Bezerra Cavalcanti Rockenbach5,
Adrian Dalca1,2,3,
Iman Aganj1,2,
Jayashree Kalpathy-Cramer1,2,
Bruce Fischl1,2,3,4,
and Robert Frost1,2
1Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 5Data Science Office, Mass General Brigham, Boston, MA, United States Keywords: Artifacts, Artifacts, Motion artifact, Image Quality, Neuroimaging Motion artifacts can negatively impact diagnosis, patient experience, and radiology workflow especially when a patient recall is required. Detecting motion artifacts while the patient is still in the scanner could potentially improve workflow and reduce costs by enabling immediate corrective action. We demonstrate in a clinical k-space dataset that using cross-correlation between adjacent phase-encoding lines can detect motion artifacts directly from raw k-space in multi-shot multi-slice scans. We train a split-attention residual network to examine the performance in predicting motion artifact severity. The network is trained on simulated data and tested on real clinical data. |
|
3426.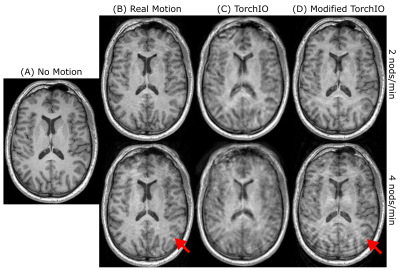 |
18 |
Large-scale simulation of rigid head motion artifacts in whole
brain MRI data and the impact on automatic cortical segmentation
Hampus Olsson1,
Jason Michael Millward1,2,
Ludger Starke1,
Tobias Klein1,
Wenli Xu1,
Sabrina Klix1,
Christoph Lippert3,4,
Thoralf Niendorf1,2,
and Sonia Waiczies1,2
1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Experimental and Clinical Research Center, a joint cooperation between the Charitë Medical Faculty and the Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 3Digital Health & Machine Learning Research Group, Hasso Plattnet Institut for Digital Engineering, Potsdam, Germany, 4Hasso Plattner Institute for Digital Health at Mount Sinai, Icahn School of Medicine at Mount Sinai, New York, NY, United States Keywords: Artifacts, Artifacts MRI datasets from epidemiological studies such as the German National Cohort (GNC) are increasingly used in machine learning. These datasets show a lower prevalence of motion artifacts than encountered in clinical practice. We simulated realistic motion artifacts caused by rigid head motion on the GNC MR data (50 subjects) by modifying the TorchIO data augmentation framework. Five levels of artifact severity were simulated. We benchmarked our results to empirical measurements using the standard GNC MPRAGE imaging protocol. The robustness to motion of FreeSurfer and SynthSeg for cortical segmentation was investigated, indicating an improved performance using SynthSeg. |
|
3427.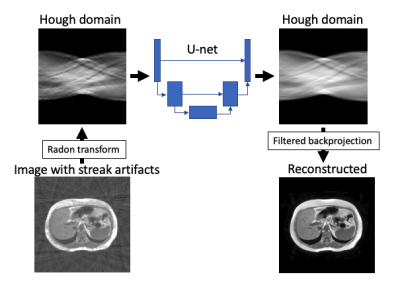 |
19 |
Streak Artifact Reduction using Convolutional Neural Network:
Clinical Feasibility Study in Liver MR Imaging
Satoshi Funayama1,
Shintaro Ichikawa1,
Yukichi Tanahashi1,
Takanobu Ikeda1,
Koh Kubota1,
Masaya Kutsuna1,
and Satoshi Goshima1
1Hamamatsu University School of Medicine, Hamamatsu, Japan Keywords: Artifacts, Machine Learning/Artificial Intelligence, Liver, Motion Correction Radial sampling enables free free-breathing abdominal MR imaging. Meanwhile, while it suffers from streak artifacts. We propose streak artifact reduction using convolutional neural network (SARC) which utilize Hough domain. In Hough domain, a streak becomes like a dot which is more localized compared with image domain. The network was trained in end-to-end manner. The SARC was show better image quality in objective image quality metrics and visual evaluation by a radiologist. SARC showed feasibility for clinical MR imaging. |
|
3428.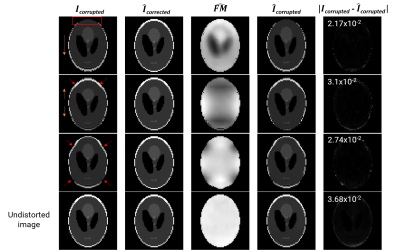 |
20 |
GDCNet: deep learning model for self-consistent geometric
distortion correction of EPI images without a field map
Marina Manso Jimeno1,2,
John Thomas Vaughan1,2,
and Sairam Geethanath2,3
1Department of Biomedical Engineering, Columbia University, New York, NY, United States, 2Columbia Magnetic Resonance Research Center, Columbia University, New York, NY, United States, 3The Biomedical Engineering Institute (Department of Diagnostic, Molecular and Interventional Radiology), Icahn School of Medicine at Mount Sinai, New York, NY, United States Keywords: Artifacts, fMRI, Geometrid Distortion, B0 inhomogeneity GDCNet is a “self-consistent” deep learning (DL) model for distortion correction of EPI fMRI images and field map estimation. It only requires the EPI images for correction, saving acquisition time and avoiding motion-related correction errors. The two supervised U-Nets for forward modelling and distortion correction have been tested in silico and in vivo on a publicly-available and a prospectively-acquired dataset. The in silico models demonstrated generalization capabilities and achieved a mean RMSE of 2.56 x10-2 as self-conistency metric. Inference in vivo showed modest correction in the prefrontal cortex and similar estimated field map compared to the acquired ground truth. |
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.