Other
Clinical Translation Challenge: Unmet Needs
ISMRM & ISMRT Annual Meeting & Exhibition • 04-09 May 2024 • Singapore

| 13:30 |
Introduction |
|
| 13:40 |
Description of Needs: Parkinson's Disease
Rahul Guarav1,
Kelly Leyden2
1, 2
|
|
| 13:44 |
9020.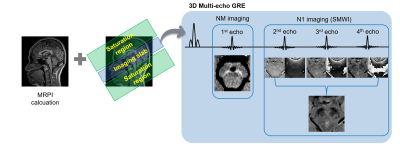 |
Simultaneous neuromelanin and nigrosome1 imaging using a single
3D multi-echo GRE sequence
Sung-Min Gho1,
Hwan Heo1,
A Leum Lee2,
and Jeongwon Jo1
1Heuron, Seoul, Korea, Republic of, 2Department of Radiology, Soonchunhyang University Bucheon Hospital, Bucheon, Korea, Republic of Motivation: Neuromelanin and nigrosome1 imaging are each instrumental in diagnosing Parkinson's disease but have been utilized separately due to constraints like extended scan time. Goal(s): To propose a 3D multi-echo GRE sequence for simultaneous imaging of neuromelanin and nigrosome1 that can be executed in a clinical setting (~5min). Approach: The previously suggested protocols for neuromelanin and nigrosome1 imaging were modified. DL-based analyses are employed for the automated detection and segmentation of neuromelanin, and for identifying the nigrosome1 regions. Results: The proposed method yields reliable estimates of neuromelanin-related volumes and identifies the nigrosome1 regions within a clinically acceptable scan time. Impact: A simultaneous neuromelanin and nigrosome1 imaging protocol was implemented within a practically feasible scan time. It achieved robust visualization of the loss of the swallow tail sign and demonstrated strong sensitivity to changes in the neuromelanin signal. |
| 13:53 |
4384.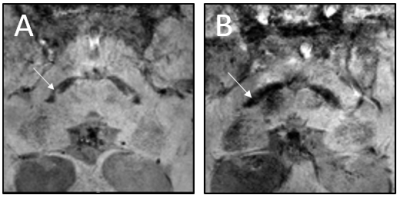 |
Accuracy of AI-driven susceptibility map-weighted MRI analyses
to differentiate neurodegenerative from non-neurodegenerative
parkinsonism
Elon D. Wallert1,
Elsmarieke van de Giessen2,
Martijn Beudel3,
Dong Hoon Shin4,5,
Tom van Mierlo6,
Jeroen Blankevoort7,
Henk W. Berendse8,
Rob M.A. de Bie3,
and Jan Booij1
1Department of Radiology and Nuclear medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 2Department of Radiology and Nuclear medicine, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands, 3Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 4'Heuron Co., Ltd., Seoul, Korea, Republic of, 5Department of Neurology, Gachon University College of Medicine, Incheon, Korea, Republic of, 6Department of Neurology, Spaarne Gasthuis, Haarlem, Netherlands, 7Department of Neurology, Flevoziekenhuis, Almere, Netherlands, 8Department of Neurology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands Keywords: Parkinson's Disease, Parkinson's Disease Motivation: Susceptibility map-weighted imaging (SMWI) of the substantia nigra is a novel MRI sequence that has the potential to aid the diagnosis of patients with clinically uncertain parkinsonian syndromes (CUPS). Goal(s): To investigate the accuracy of AI-driven automated SMWI software in a clinically relevant population. Approach: We acquired SMWI in patients who received a dopamine transporter (DAT)-SPECT because of CUPS. The diagnostic software (Heuron IPD) results were compared with the DAT-SPECT results as a reference. Results: Preliminary analysis of 120 patients demonstrated an accuracy of 88% for the diagnostic software to differentiate neurodegenerative from non-neurodegenerative parkinsonism in patients who presented with CUPS. Impact: Susceptibility map-weighted imaging (SMWI) demonstrates a diagnostic accuracy of 88% in patients with clinically uncertain parkinsonian syndromes (CUPS). These findings are promising for the use of SWMI as diagnostic marker and warrant prospective studies in CUPS patients. |
| 14:02 |
Description of Needs: Gastrointestinal Motility
Sofieke de Jong
|
|
| 14:06 |
9021.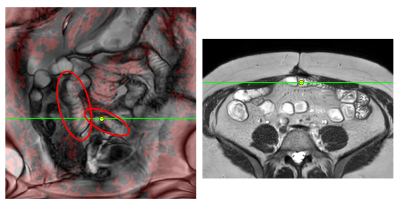 |
Challenge examples: Visualisation of shear from cine MRI for
detection of gastrointestinal adhesions
David Atkinson1 and
Stuart Taylor1
1Centre for Medical Imaging, University College London, London, United Kingdom Keywords: Gastrointestinal Motility Motivation: Further examples and methodology extending our abstract to the Unmet Needs Challenge on Gastrointestinal Motility [1]. Goal(s): Localisation of regions of bowel that are affected by adhesions. Approach: We previously proposed that regions of reduced shear may correspond to bowel motility hindered by adhesions [1]. Shear was calculated from coronal cine frames and presented as red colour overlays. Here we present further examples and add a linked-cursor that links regions of restricted motility to impressions of tethering in axial structural images, e.g. angulated bowel loops. Results: Descriptive exemplars are presented.
Impact: Adhesions cannot be directly seen in MRI but
tethering may be apparent in structural and motility images.
The proposed post-processing methods use readily available
MR sequences and Results suggest further investigation to
validate observations and to assess potential clinical
benefit. |
| 14:15 |
Description of Needs: Central Nervous System Lactate
Julie Harreld
|
|
| 14:19 |
9022.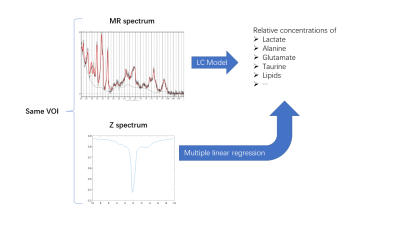 |
Calculation of metabolite concentrations using linear
combination of CEST Z-spactra -- a study on 9.4 T rodent
datasets
Yifan Li1,
Teng Gong1,
Wentao Jia2,
Yuqing Wang3,
Lele Ma1,
Nan Gao1,
and Xiaolei Song1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 2Department of lnformation Science and Technology, Northwest University, Xi'an, China, 3Nonhuman Primate Research Center, Tsinghua University, Beijing, China Motivation: Lactate can be detected by both MRS and CEST. Compared to MRS, CEST imaging is faster due to its high sensitivity, but the low specificity limits it to qualitative study. Goal(s): If deriving reliable quantitative maps from CEST Z-spectra is possible, the lactate imaging can be accelarated significantly. Approach: On our rodent MRS and CEST dataset, we calculated the concentrations of lactate (and other metabolites) from single voxel MRS data as gold standard, and performed regression between them with the mean CEST Z-spectra of the same VOI. Results: Preliminary results show lactate concentrations can be estimated by linear combination of Z-spectra. Impact: Our preliminery work may provide a new insight into faster lactate imaging, that is, using a faster but less quantitative modality like CEST to acquire images and building the correlation of it with more reliable quantitative values. |
| 14:28 |
Description of Needs: Amyloid Tau Proteins
Nandor Pinter
|
|
| 14:32 |
0961.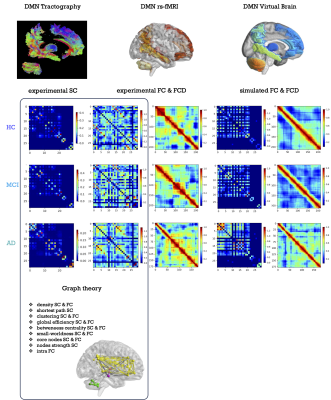 |
Topological network analysis and virtual brain modelling
combined to portray subject-specific profiles of dementia
stages.
Anita Monteverdi1,
Fulvia Palesi1,2,
Sofia Manzon2,
Francesca Conca3,
Laura Mazzocchi4,
Matteo Cotta Ramusino5,
Eleonora Lupi2,
Marialaura De Grazia2,
Roberta Maria Lorenzi2,
Marta Gaviraghi2,
Lisa Farina3,
Alfredo Costa2,5,
Anna Pichiecchio2,4,
Stefano F. Cappa3,6,
Claudia A.M. Gandini Wheeler-Kingshott1,2,7,
and Egidio D'Angelo1,2
1Digital Neuroscience Centre, IRCCS Mondino Foundation, Pavia, Italy, 2Brain and Behavioral Sciences, University of Pavia, Pavia, Italy, 3IRCCS Mondino Foundation, Pavia, Italy, 4Advanced Imaging and Artificial Intelligence Center, IRCCS Mondino Foundation, Pavia, Italy, 5Unit of Behavioral Neurology, IRCCS Mondino Foundation, Pavia, Italy, 6University Institute of Advanced Studies (IUSS), Pavia, Italy, 7NMR Research Unit, Queen Square Multiple Sclerosis Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, London, United Kingdom Keywords: Alzheimer's Disease, Modelling, Virtual Brain modelling, biomarkers, brain dynamics, excitatory/inhibitory balance Motivation: The high level of heterogeneity typical of mild cognitive impairment (MCI) condition currently hinders the selection of a personalized effective therapy. Goal(s): Our goal is to obtain a personalized profile exploring not only structural and functional topology but also diving in subject-specific physiological parameters. Approach: Starting from structural and functional connectomes, we combined graph theoretical analysis with virtual brain models in the default mode network of healthy subjects, MCI and Alzheimer's disease patients. Results: Our results offer a detailed description of alterations at single-subject level, illustrating differences between dementia stages based on topology and subject-specific physiological parameters. Impact: The personalized profile obtained combining graph theory and virtual brain models portray dementia stages at single-subject level, capturing the wide heterogeneity of mild cognitive impairment and opening new perspectives for personalized effective interventions. |
| 14:41 |
9023.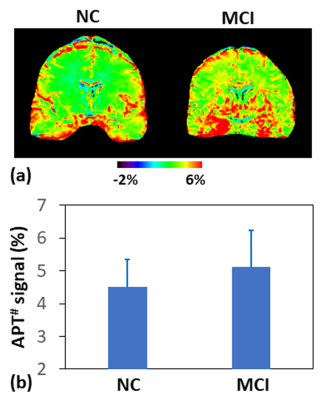 |
Protein-based Amide Proton Transfer MRI Signal as a biomarker
for AD Immunotherapy Monitoring
Jinyuan Zhou1,
Jingpu Wu2,
Jieru Wan2,
Munendra Singh2,
Puyang Wang2,
Hye-Young Heo2,
and Shanshan Jiang2
1Department of Radiology, Johns Hopkins University, Baltimore, MD, United States, 2Johns Hopkins University, Baltimore, MD, United States Motivation: Rigorous monitoring is clearly needed to fully evaluate efficacy of new anti-amyloid therapeutics against AD. Goal(s): To evaluate the value of protein-based APT MRI in monitoring AD immunotherapy efficacy and characterizing adverse events. Approach: Both animal AD models and human subjects were studied, and a novel APT acquisition and quantitative analysis approach (EMR-APT) was used. Results: The average APT signals were significantly higher in AD mice than in wild-type controls. Similarly, the MCI patients demonstrated significantly higher APT signals, compared to the normal controls. Impact: A unique and innovative biomarker-stratified approach developed in this work will aid in assessing treatment efficacy accurately and identifying adverse events early. |
| 14:50 |
9024.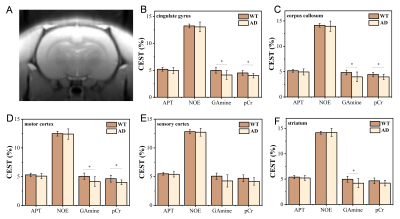 |
Early detection of Alzheimer’s disease in a transgenic rat model
using CEST MRI
Teng Gong1,
Nan Gao1,
Wentao Jia2,
Yifan Li1,
and Xiaolei Song1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 2Department of lnformation Science and Technology, Northwest University, Xi'an, China Motivation: There is a lack of effective MR tool for early diagnosis of AD. Goal(s): To assess the value of CEST MRI for early detection of AD using a transgenic rat model. Approach: CEST MRI scans for 10 AD rats and 9 age-matched wide-type (WT) controls were performed on 9.4T animal MR scanner. CEST signals were quantified using Lorentzian Difference, with signals at multiple frequency offsets quantified. Results: Compared with WT rats, pCr and G-amine signals in several brain regions of AD rats were significantly decreased, which is expected to be used as biomarkers for early detection of AD. Impact: The study provides evidence for early detection of AD in transgenic rat brains using in vivo CEST MRI and may promote clinical translation. |
| 14:59 |
3911.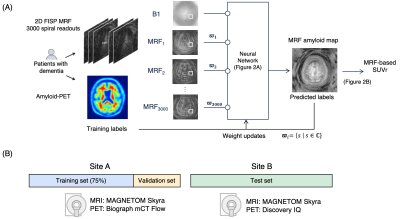 |
MR fingerprinting for quantification of brain amyloid burden:
from development to prospective multi-site external validation
Shohei Fujita1,2,3,4,
Yasutaka Fushimi5,
Yujiro Otsuka1,6,7,
Katsutoshi Murata8,
Guido Buonincontri9,
Gregor Koerzdoerfer10,
Mathias Nittka9,
Issei Fukunaga1,
Kaito Takabayashi1,
Yumiko Motoi11,12,
Madoka Nakajima12,13,
Koji Murakami14,
Atsushi Shima15,
Manabu Kubota16,
Berkin Bilgic3,4,17,
Koji Kamagata1,
Nobukatsu Sawamoto18,
Osamu Abe2,
Yuji Nakamoto5,
and Shigeki Aoki1
1Dept. of Radiology, Juntendo University, Tokyo, Japan, 2Dept. of Radiology, The University of Tokyo, Tokyo, Japan, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States, 4Dept. of Radiology, Harvard Medical School, Boston, MA, United States, 5Dept. of Diagnostic Imaging and Nuclear Medicine, Kyoto University, Kyoto, Japan, 6Milliman Inc, Tokyo, Japan, 7Plusman LLC, Tokyo, Japan, 8Siemens Healthcare Japan KK, Tokyo, Japan, 9Siemens Healthcare GmbH, Erlangen, Germany, 10Siemens Medical Solutions, New York, NY, United States, 11Dept. of Neurology, Juntendo University, Tokyo, Japan, 1212. Medical Center for Dementia, Juntendo University, Tokyo, Japan, 13Dept. of Neurosurgery, Juntendo University, Tokyo, Japan, 14Division of Nuclear Medicine, Dept. of Radiology, Juntendo University, Tokyo, Japan, 15Department of Regenerative Systems Neuroscience, Human Brain Research Center, Kyoto University, Kyoto, Japan, 16Dept. of Psychiatry, Kyoto University, Kyoto, Japan, 17Harvard/MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 18Dept. of Human Health Sciences, Kyoto University, Kyoto, Japan Keywords: Alzheimer's Disease, Alzheimer's Disease, Biomarker Motivation: A non-invasive amyloid beta (Aβ) imaging technique is needed for objective diagnosis and treatment monitoring of Alzheimer’s disease. Goal(s): To develop and validate an MRF-based method quantifying brain Aβ. Approach: A framework with efficient MRF data acquisition, neural network decoding, and atlas-based segmentation was implemented. A prospective analysis was conducted on external dataset to evaluate its generalizability, repeatability, and correlation with Aβ-PET measurements and clinical cognitive function tests. Results: The method showed high repeatability (CV<2%), significant correlation with Aβ-PET measurements and Montreal Cognitive Assessment test (p=0.015 and 0.020, respectively), and discriminated subject-level Aβ positivity with an AUC of 0.84 on external test set. Impact: The proposed framework is compatible with clinical 3T MRI and offers ‘one-stop’ examination in 10 minutes for patients with cognitive decline by providing structural MRI and Aβ-quantification. Its non-invasive nature facilitates longitudinal evaluation and correlates with Aβ-PET and cognitive function. |
| 15:08 |
Discussion & Conclusion |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.